Harnessing Fluorine-Sulfur Contacts and Multipolar Interactions for the Design of p53 Mutant Y220C Rescue Drugs
- PMID: 27267810
- PMCID: PMC4994063
- DOI: 10.1021/acschembio.6b00315
Harnessing Fluorine-Sulfur Contacts and Multipolar Interactions for the Design of p53 Mutant Y220C Rescue Drugs
Abstract
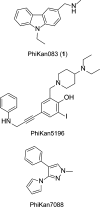
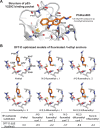
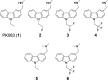
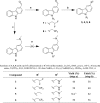
Many oncogenic mutants of the tumor suppressor p53 are conformationally unstable, including the frequently occurring Y220C mutant. We have previously developed several small-molecule stabilizers of this mutant. One of these molecules, PhiKan083, 1-(9-ethyl-9H-carbazole-3-yl)-N-methylmethanamine, binds to a mutation-induced surface crevice with a KD = 150 μM, thereby increasing the melting temperature of the protein and slowing its rate of aggregation. Incorporation of fluorine atoms into small molecule ligands can substantially improve binding affinity to their protein targets. We have, therefore, harnessed fluorine-protein interactions to improve the affinity of this ligand. Step-wise introduction of fluorines at the carbazole ethyl anchor, which is deeply buried within the binding site in the Y220C-PhiKan083 complex, led to a 5-fold increase in affinity for a 2,2,2-trifluoroethyl anchor (ligand efficiency of 0.3 kcal mol(-1) atom(-1)). High-resolution crystal structures of the Y220C-ligand complexes combined with quantum chemical calculations revealed favorable interactions of the fluorines with protein backbone carbonyl groups (Leu145 and Trp146) and the sulfur of Cys220 at the mutation site. Affinity gains were, however, only achieved upon trifluorination, despite favorable interactions of the mono- and difluorinated anchors with the binding pocket, indicating a trade-off between energetically favorable protein-fluorine interactions and increased desolvation penalties. Taken together, the optimized carbazole scaffold provides a promising starting point for the development of high-affinity ligands to reactivate the tumor suppressor function of the p53 mutant Y220C in cancer cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Zhou Y.; Wang J.; Gu Z.; Wang S.; Zhu W.; Acena J. L.; Soloshonok V. A.; Izawa K.; Liu H. (2016) Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 116, 422–518. 10.1021/acs.chemrev.5b00392. - DOI - PubMed
-
- Kirk K. L. (2006) Fluorine in medicinal chemistry: Recent therapeutic applications of fluorinated small molecules. J. Fluorine Chem. 127, 1013–1029. 10.1016/j.jfluchem.2006.06.007. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

