A Novel HSP90 Inhibitor-Drug Conjugate to SN38 Is Highly Effective in Small Cell Lung Cancer
- PMID: 27267850
- PMCID: PMC5065742
- DOI: 10.1158/1078-0432.CCR-15-3068
A Novel HSP90 Inhibitor-Drug Conjugate to SN38 Is Highly Effective in Small Cell Lung Cancer
Abstract
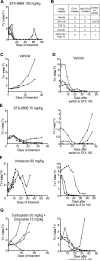
Purpose: Small cell lung cancer (SCLC) is a highly aggressive disease representing 12% to 13% of total lung cancers, with median survival of <2 years. No targeted therapies have proven effective in SCLC. Although most patients respond initially to cytotoxic chemotherapies, resistance rapidly emerges, response to second-line agents is limited, and dose-limiting toxicities (DLT) are a major issue. This study performs preclinical evaluation of a new compound, STA-8666, in SCLC.
Experimental design: To avoid DLT for useful cytotoxic agents, the recently developed drug STA-8666 combines a chemical moiety targeting active HSP90 (concentrated in tumors) fused via cleavable linker to SN38, the active metabolite of irinotecan. We compare potency and mechanism of action of STA-8666 and irinotecan in vitro and in vivo RESULTS: In two SCLC xenograft and patient-derived xenograft models, STA-8666 was tolerated without side effects up to 150 mg/kg. At this dose, STA-8666 controlled or eliminated established tumors whether used in a first-line setting or in tumors that had progressed following treatment on standard first- and second-line agents for SCLC. At 50 mg/kg, STA-8666 strongly enhanced the action of carboplatin. Pharmacokinetic profiling confirmed durable STA-8666 exposure in tumors compared with irinotecan. STA-8666 induced a more rapid, robust, and stable induction of cell-cycle arrest, expression of signaling proteins associated with DNA damage and cell-cycle checkpoints, and apoptosis in vitro and in vivo, in comparison with irinotecan.
Conclusions: Together, these results strongly support clinical development of STA-8666 for use in the first- or second-line setting for SCLC. Clin Cancer Res; 22(20); 5120-9. ©2016 AACR.
©2016 American Association for Cancer Research.
Figures




Comment in
-
Emerging strategies for the treatment of advanced small cell lung cancer.J Thorac Dis. 2016 Oct;8(10):E1249-E1253. doi: 10.21037/jtd.2016.10.46. J Thorac Dis. 2016. PMID: 27867600 Free PMC article. No abstract available.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

