An observational study of the use of beclomethasone dipropionate suppositories in the treatment of lower urinary tract inflammation in men
- PMID: 27267961
- PMCID: PMC4897870
- DOI: 10.1186/s12894-016-0144-8
An observational study of the use of beclomethasone dipropionate suppositories in the treatment of lower urinary tract inflammation in men
Abstract
Background: Nonbacterial prostatitis, together with chronic pelvic pain syndrome, accounts for 90-95 % of prostatitis cases. Anti-inflammatory medications are commonly used to reduce storage/inflammatory symptoms that can deteriorate quality of life. The purpose of this study was to observe the efficacy and safety of beclomethasone dipropionate rectal suppositories (Topster®) in inflammations of the lower urinary tract in men.
Methods: Patients underwent diagnostic and therapeutic protocols according to current evidence-based practice. Efficacy assessments: voiding parameters, perineal pain, International Prostate Symptom Score (IPSS), digital rectal examination (DRE). Adverse events and patient compliance were recorded throughout the study.
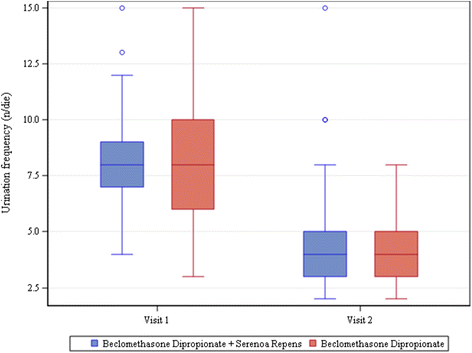
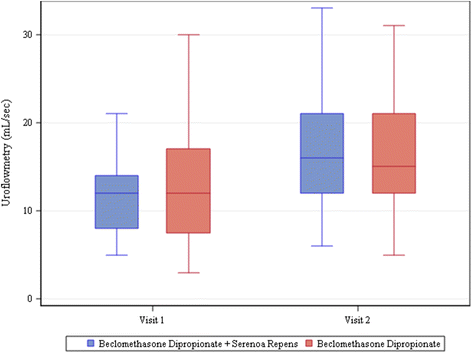
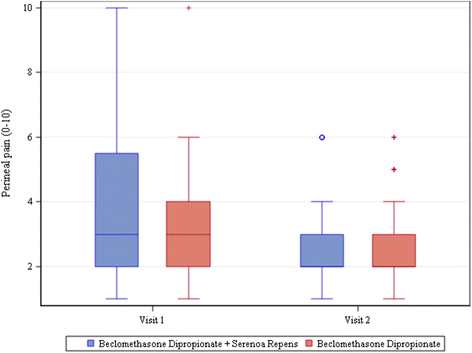
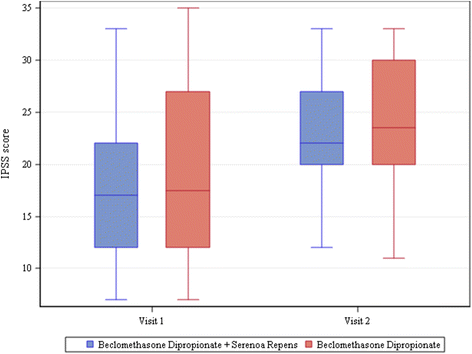
Results: One hundred eighty patients were enrolled, mean age 52 ± 14.97. Most frequent diagnosis: nonbacterial prostatitis (85 %). All patients completed visits 1 and 2. All patients were treated with beclomethasone dipropionate (BDP) suppositories, 136/180 also with Serenoa repens (SR) extract. Antibiotics were rarely required. 162/180 patients presented clinically significant improvements and terminated treatment. Mean change vs. baseline in voiding frequency: -3.55 ± 2.70 n/day in patients taking only BDP and -3.68 ± 2.81 n/day in those taking both BDP and SR (P<.0001 in both groups). Uroflowmetry improved significantly; change from baseline 3.26 ± 5.35 ml/s in BDP only group and 5.61 ± 7.32 ml/s in BDP + SR group (P = 0.0002 for BDP, P<.0001 for BDP + SR). Urine stream normal in 35 % of patients at visit 1 and 57.22 % of patients at visit 2. Mean change in perineal pain, on 0-10 VAS, -0.66 ± 2.24 for BDP only group (P = 0.0699) and -1.37 ± 2.40 for BDP + SR group (P<.0001). IPSS increased at visit 2. No adverse events were reported. For all parameters, none of the comparisons between groups was found to be statistically significant.
Conclusion: This study confirmed the drug's good safety profile. We also observed an improvement in the main storage symptoms and clinical findings associated with lower urinary tract inflammation in patients treated with beclomethasone dipropionate suppositories.
Keywords: Beclomethasone dipropionate; Lower urinary tract inflammation; Nonbacterial prostatitis.
Figures
Similar articles
-
Multicentre study on the efficacy and tolerability of an extract of Serenoa repens in patients with chronic benign prostate conditions associated with inflammation.Arch Ital Urol Androl. 2012 Jun;84(2):94-8. Arch Ital Urol Androl. 2012. PMID: 22908779 Clinical Trial.
-
Long-term safety and efficacy of a chlorofluorocarbon-free beclomethasone dipropionate extrafine aerosol.Ann Allergy Asthma Immunol. 2001 May;86(5):557-65. doi: 10.1016/S1081-1206(10)62905-5. Ann Allergy Asthma Immunol. 2001. PMID: 11379808 Clinical Trial.
-
Randomised clinical trial: preventive treatment with topical rectal beclomethasone dipropionate reduces post-radiation risk of bleeding in patients irradiated for prostate cancer.Aliment Pharmacol Ther. 2011 Sep;34(6):628-37. doi: 10.1111/j.1365-2036.2011.04780.x. Epub 2011 Jul 25. Aliment Pharmacol Ther. 2011. PMID: 21790680 Clinical Trial.
-
The safety of beclomethasone dipropionate in the treatment of ulcerative colitis.Expert Opin Drug Saf. 2018 Sep;17(9):963-969. doi: 10.1080/14740338.2018.1510914. Epub 2018 Aug 21. Expert Opin Drug Saf. 2018. PMID: 30101623 Review.
-
Prostatitis and urinary tract infection in men: what's new; what's true?Am J Med. 1999 Mar;106(3):327-34. doi: 10.1016/s0002-9343(99)00017-0. Am J Med. 1999. PMID: 10190383 Review.
Cited by
-
Recent advances in managing chronic prostatitis/chronic pelvic pain syndrome.F1000Res. 2017 Sep 25;6:F1000 Faculty Rev-1747. doi: 10.12688/f1000research.10558.1. eCollection 2017. F1000Res. 2017. PMID: 29034074 Free PMC article. Review.
References
-
- Bartoletti R, Cai T, Mondaini N, et al. Prevalence, incidence estimation, risk factors and characterization of chronic prostatitis/chronic pelvic pain syndrome in urological hospital outpatients in Italy: results of a multicenter case–control observational study. J Urol. 2007;178(6):2411–5. doi: 10.1016/j.juro.2007.08.046. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

