Hypermutation and unique mutational signatures of occupational cholangiocarcinoma in printing workers exposed to haloalkanes
- PMID: 27267998
- PMCID: PMC4967217
- DOI: 10.1093/carcin/bgw066
Hypermutation and unique mutational signatures of occupational cholangiocarcinoma in printing workers exposed to haloalkanes
Abstract
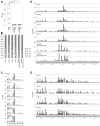
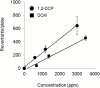
Cholangiocarcinoma is a relatively rare cancer, but its incidence is increasing worldwide. Although several risk factors have been suggested, the etiology and pathogenesis of the majority of cholangiocarcinomas remain unclear. Recently, a high incidence of early-onset cholangiocarcinoma was reported among the workers of a printing company in Osaka, Japan. These workers underwent high exposure to organic solvents, mainly haloalkanes such as 1,2-dichloropropane (1,2-DCP) and/or dichloromethane. We performed whole-exome analysis on four cases of cholangiocarcinoma among the printing workers. An average of 44.8 somatic mutations was detected per Mb in the genome of the printing workers' cholangiocarcinoma tissues, approximately 30-fold higher than that found in control common cholangiocarcinoma tissues. Furthermore, C:G-to-T:A transitions with substantial strand bias as well as unique trinucleotide mutational changes of GpCpY to GpTpY and NpCpY to NpTpY or NpApY were predominant in all of the printing workers' cholangiocarcinoma genomes. These results were consistent with the epidemiological observation that they had been exposed to high concentrations of chemical compounds. Whole-genome analysis of Salmonella typhimurium strain TA100 exposed to 1,2-DCP revealed a partial recapitulation of the mutational signature in the printing workers' cholangiocarcinoma. Although our results provide mutational signatures unique to occupational cholangiocarcinoma, the underlying mechanisms of the disease should be further investigated by using appropriate model systems and by comparison with genomic data from other cancers.
© The Author 2016. Published by Oxford University Press.
Figures

References
-
- Matsuda A., et al. (2014) Cancer incidence and incidence rates in Japan in 2008: a study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J. Clin. Oncol., 44, 388–396. - PubMed
-
- Everhart J.E., et al. (2009) Burden of digestive diseases in the United States Part III: liver, biliary tract, and pancreas. Gastroenterology, 136, 1134–1144. - PubMed
-
- Khan S.A., et al. (2005) Cholangiocarcinoma. Lancet, 366, 1303–1314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

