Dimethyl fumarate restores apoptosis sensitivity and inhibits tumor growth and metastasis in CTCL by targeting NF-κB
- PMID: 27268084
- PMCID: PMC5026464
- DOI: 10.1182/blood-2016-01-694117
Dimethyl fumarate restores apoptosis sensitivity and inhibits tumor growth and metastasis in CTCL by targeting NF-κB
Abstract
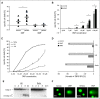
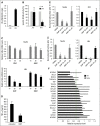
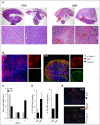
Despite intensive efforts in recent years, a curative therapy for cutaneous T-cell lymphoma (CTCL) has not yet been developed. Therefore, the establishment of new therapeutic approaches with higher efficacy rates and milder side effects is strongly desired. A characteristic feature of the malignant T-cell population in CTCL is resistance toward cell death resulting from constitutive NF-κB activation. Therefore, NF-κB-dependent cell death resistance represents an interesting therapeutic target in CTCL because an NF-κB-directed therapy would leave bystander T cells widely unaffected. We investigated the effects of dimethyl fumarate (DMF) on CTCL cells in vitro and in vivo. DMF induced cell death in primary patient-derived CD4(+) cells and CTCL cell lines, but hardly in T cells from healthy donors. DMF-induced cell death was linked specifically to NF-κB inhibition. To study the impact of DMF in vivo, we developed 2 CTCL xenograft mouse models with different cutaneous localizations of the T-cell infiltrate. DMF treatment delayed the growth of CTCL tumors and prevented formation of distant metastases. In addition, DMF induced increased cell death in primary CTCL tumors and in liver metastases. In summary, DMF treatment represents a remarkable therapeutic option in CTCL because it restores CTCL apoptosis in vitro and in preclinical models in vivo and prevents spreading of the disease to distant sites. DMF treatment is of particular promise in CTCL because DMF is already in successful clinical use in the treatment of psoriasis and multiple sclerosis allowing fast translation into clinical studies in CTCL.
© 2016 by The American Society of Hematology.
Figures


Comment in
-
DMF: a promising therapeutic option in CTCL.Blood. 2016 Aug 11;128(6):745-6. doi: 10.1182/blood-2016-06-722462. Blood. 2016. PMID: 27516426 Free PMC article.
References
-
- Olsen E, Vonderheid E, Pimpinelli N, et al. ISCL/EORTC. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110(6):1713–1722. - PubMed
-
- Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sézary syndrome. Blood. 2009;114(20):4337–4353. - PubMed
-
- Wilcox RA. Cutaneous T-cell lymphoma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(8):837–851. - PubMed
-
- Olsen EA, Rook AH, Zic J, et al. Sézary syndrome: immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the United States Cutaneous Lymphoma Consortium (USCLC). J Am Acad Dermatol. 2011;64(2):352–404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

