Adult human megakaryocyte-erythroid progenitors are in the CD34+CD38mid fraction
- PMID: 27268089
- PMCID: PMC4990855
- DOI: 10.1182/blood-2016-01-693705
Adult human megakaryocyte-erythroid progenitors are in the CD34+CD38mid fraction
Erratum in
-
Sanada C, Xavier-Ferrucio J, Lu Y-C, et al. Adult human megakaryocyte-erythroid progenitors are in the CD34+CD38mid fraction. Blood. 2016;128(7):923-933.Blood. 2017 Feb 16;129(7):919. doi: 10.1182/blood-2017-01-760751. Blood. 2017. PMID: 28209755 Free PMC article. No abstract available.
Abstract
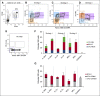
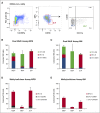
Bipotent megakaryocyte/erythroid progenitors (MEPs) give rise to progeny limited to the megakaryocyte (Mk) and erythroid (E) lineages. We developed a novel dual-detection functional in vitro colony-forming unit (CFU) assay for single cells that differentiates down both the Mk and E lineages (CFU-Mk/E), which allowed development and validation of a novel purification strategy for the identification and quantitation of primary functional human MEPs from granulocyte colony-stimulating factor-mobilized peripheral blood and bone marrow. Applying this assay to fluorescence-activated cell sorter-sorted cell populations, we found that the Lin(-)CD34(+)CD38(mid)CD45RA(-)FLT3(-)MPL(+)CD36(-)CD41(-) population is much more highly enriched for bipotent MEPs than any previously reported subpopulations. We also developed purification strategies for primary human lineage-committed Mk and E progenitors identified as CFU-Mk and burst forming unit-E. Comparative expression analyses in MEP, MkP, and ErP populations revealed differential expression of MYB We tested whether alterations in MYB concentration affect the Mk-E fate decision at the single cell level in MEPs and found that short hairpin RNA-mediated MYB knockdown promoted commitment of MEPs to the Mk lineage, further defining its role in MEP lineage fate. There are numerous applications for these novel enrichment strategies, including facilitating mechanistic studies of MEP lineage commitment, improving approaches for in vitro expansion of Mk and E cells, and developing improved therapies for benign and malignant hematologic disease.
© 2016 by The American Society of Hematology.
Figures





Comment in
-
The road not taken?Blood. 2016 Aug 18;128(7):886-8. doi: 10.1182/blood-2016-07-722413. Blood. 2016. PMID: 27539995 No abstract available.
References
-
- Martin P, Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982;216(4551):1233–1235. - PubMed
-
- Greenberg SM, Rosenthal DS, Greeley TA, Tantravahi R, Handin RI. Characterization of a new megakaryocytic cell line: the Dami cell. Blood. 1988;72(6):1968–1977. - PubMed
-
- Sato T, Fuse A, Eguchi M, et al. Establishment of a human leukaemic cell line (CMK) with megakaryocytic characteristics from a Down’s syndrome patient with acute megakaryoblastic leukaemia. Br J Haematol. 1989;72(2):184–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

