CASEIN KINASE1-LIKE PROTEIN2 Regulates Actin Filament Stability and Stomatal Closure via Phosphorylation of Actin Depolymerizing Factor
- PMID: 27268429
- PMCID: PMC4944410
- DOI: 10.1105/tpc.16.00078
CASEIN KINASE1-LIKE PROTEIN2 Regulates Actin Filament Stability and Stomatal Closure via Phosphorylation of Actin Depolymerizing Factor
Abstract
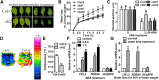
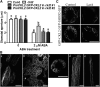
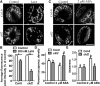
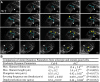
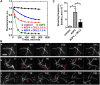
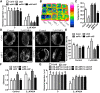
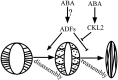
The opening and closing of stomata are crucial for plant photosynthesis and transpiration. Actin filaments undergo dynamic reorganization during stomatal closure, but the underlying mechanism for this cytoskeletal reorganization remains largely unclear. In this study, we identified and characterized Arabidopsis thaliana casein kinase 1-like protein 2 (CKL2), which responds to abscisic acid (ABA) treatment and participates in ABA- and drought-induced stomatal closure. Although CKL2 does not bind to actin filaments directly and has no effect on actin assembly in vitro, it colocalizes with and stabilizes actin filaments in guard cells. Further investigation revealed that CKL2 physically interacts with and phosphorylates actin depolymerizing factor 4 (ADF4) and inhibits its activity in actin filament disassembly. During ABA-induced stomatal closure, deletion of CKL2 in Arabidopsis alters actin reorganization in stomata and renders stomatal closure less sensitive to ABA, whereas deletion of ADF4 impairs the disassembly of actin filaments and causes stomatal closure to be more sensitive to ABA Deletion of ADF4 in the ckl2 mutant partially recues its ABA-insensitive stomatal closure phenotype. Moreover, Arabidopsis ADFs from subclass I are targets of CKL2 in vitro. Thus, our results suggest that CKL2 regulates actin filament reorganization and stomatal closure mainly through phosphorylation of ADF.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures

Comment in
-
Battening Down the Hatches: A Role for CASEIN KINASE1-LIKE PROTEIN2 in Stomatal Closure.Plant Cell. 2016 Jun;28(6):1236. doi: 10.1105/tpc.16.00462. Epub 2016 Jun 10. Plant Cell. 2016. PMID: 27288223 Free PMC article. No abstract available.
Similar articles
-
Arabidopsis ADF5 promotes stomatal closure by regulating actin cytoskeleton remodeling in response to ABA and drought stress.J Exp Bot. 2019 Jan 7;70(2):435-446. doi: 10.1093/jxb/ery385. J Exp Bot. 2019. PMID: 30476276 Free PMC article.
-
The PYR-PP2C-CKL2 module regulates ABA-mediated actin reorganization during stomatal closure.New Phytol. 2022 Mar;233(5):2168-2184. doi: 10.1111/nph.17933. Epub 2022 Jan 7. New Phytol. 2022. PMID: 34932819
-
A Role for Plant KASH Proteins in Regulating Stomatal Dynamics.Plant Physiol. 2020 Feb;182(2):1100-1113. doi: 10.1104/pp.19.01010. Epub 2019 Nov 25. Plant Physiol. 2020. PMID: 31767690 Free PMC article.
-
Actin branches out to link pathogen perception and host gene regulation.Plant Signal Behav. 2013 Mar;8(3):e23468. doi: 10.4161/psb.23468. Epub 2013 Jan 18. Plant Signal Behav. 2013. PMID: 23333960 Free PMC article. Review.
-
Controlling the Gate: The Functions of the Cytoskeleton in Stomatal Movement.Front Plant Sci. 2022 Feb 23;13:849729. doi: 10.3389/fpls.2022.849729. eCollection 2022. Front Plant Sci. 2022. PMID: 35283892 Free PMC article. Review.
Cited by
-
Phosphorylation of GENOMES UNCOUPLED 4 Alters Stimulation of Mg Chelatase Activity in Angiosperms.Plant Physiol. 2016 Nov;172(3):1578-1595. doi: 10.1104/pp.16.01036. Epub 2016 Sep 29. Plant Physiol. 2016. PMID: 27688621 Free PMC article.
-
Plant Actin-Depolymerizing Factors Possess Opposing Biochemical Properties Arising from Key Amino Acid Changes throughout Evolution.Plant Cell. 2017 Feb;29(2):395-408. doi: 10.1105/tpc.16.00690. Epub 2017 Jan 25. Plant Cell. 2017. PMID: 28123105 Free PMC article.
-
Arabidopsis ADF5 Acts as a Downstream Target Gene of CBFs in Response to Low-Temperature Stress.Front Cell Dev Biol. 2021 Jan 28;9:635533. doi: 10.3389/fcell.2021.635533. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33585491 Free PMC article.
-
SCAB1 coordinates sequential Ca2+ and ABA signals during osmotic stress induced stomatal closure in Arabidopsis.Sci China Life Sci. 2024 Jan;67(1):1-18. doi: 10.1007/s11427-023-2480-4. Epub 2023 Dec 18. Sci China Life Sci. 2024. PMID: 38153680
-
The rubber tree kinome: Genome-wide characterization and insights into coexpression patterns associated with abiotic stress responses.Front Plant Sci. 2023 Feb 7;14:1068202. doi: 10.3389/fpls.2023.1068202. eCollection 2023. Front Plant Sci. 2023. PMID: 36824205 Free PMC article.
References
-
- Agnew B.J., Minamide L.S., Bamburg J.R. (1995). Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J. Biol. Chem. 270: 17582–17587. - PubMed
-
- Allwood E.G., Smertenko A.P., Hussey P.J. (2001). Phosphorylation of plant actin-depolymerising factor by calmodulin-like domain protein kinase. FEBS Lett. 499: 97–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

