Enantioselective Intermolecular [2 + 2] Photocycloaddition Reactions of 2(1H)-Quinolones Induced by Visible Light Irradiation
- PMID: 27268908
- PMCID: PMC4929526
- DOI: 10.1021/jacs.6b03221
Enantioselective Intermolecular [2 + 2] Photocycloaddition Reactions of 2(1H)-Quinolones Induced by Visible Light Irradiation
Abstract
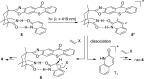
In the presence of a chiral thioxanthone catalyst (10 mol %) the title compounds underwent a clean intermolecular [2 + 2] photocycloaddition with electron-deficient olefins at λ = 419 nm. The reactions not only proceeded with excellent regio- and diastereoselectivity but also delivered the respective cyclobutane products with significant enantiomeric excess (up to 95% ee). Key to the success of the reactions is a two-point hydrogen bonding between quinolone and catalyst enabling efficient energy transfer and high enantioface differentiation. Preliminary work indicated that solar irradiation can be used for this process and that the substrate scope can be further expanded to isoquinolones.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



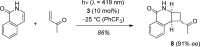
References
-
-
Reviews:
- Bauslaugh P. G. Synthesis 1970, 287–300. 10.1055/s-1970-21606. - DOI
- Baldwin S. W. Org. Photochem. 1981, 5, 123–225.
- Crimmins M. T. Chem. Rev. 1988, 88, 1453–1473. 10.1021/cr00090a002. - DOI
- Becker D.; Haddad N. Org. Photochem. 1989, 10, 1–162.
- Crimmins M. T.; Reinhold T. L. Org. React. 1993, 44, 297–588. 10.1002/0471264180.or044.02. - DOI
- Mattay J.; Conrads R.; Hoffmann R.. [2 + 2] Photocycloadditions of α,β-Unsaturated Carbonyl Compounds. In Methoden der Organischen Chemie (Houben-Weyl), 4th ed.; Vol. E 21c; Helmchen G., Hoffmann R. W., Mulzer J., Schaumann E., Eds.; Thieme: Stuttgart, 1995; pp 3087–3132.
- Pete J.-P. Adv. Photochem. 1996, 21, 135–216. 10.1002/9780470133521.ch2. - DOI
- Fleming S. A.; Bradford C. L.; Gao J. J.. Regioselective and Stereoselective [2 + 2] Photocycloadditions. In Organic Photochemistry, Molecular and Supramolecular Photochemistry, Vol. 1; Ramamurthy V., Schanze K. S., Eds.; Dekker: New York, 1997; pp 187–244.
- Bach T. Synthesis 1998, 683–703. 10.1055/s-1998-2054. - DOI
- Margaretha P.Photocycloaddition of Cycloalk-2-enones to Alkenes. In Synthetic Organic Photochemistry, Molecular and Supramolecular Photochemistry, Vol. 12; Griesbeck A. G., Mattay J., Eds.; Dekker: New York, 2005; pp 211–237.
- Hehn J. P.; Müller C.; Bach T.. Formation of a Four-Membered Ring: From a Carbonyl-Conjugated Alkene. In Handbook of Synthetic Photochemistry; Albini A., Fagnoni M., Eds.; Wiley-VCH: Weinheim, 2010; pp 171–215.
- Poplata S.; Tröster A.; Zou Y.-Q.; Bach T.. Chem. Rev. 2016, 116, in press, doi: 10.1021/acs.chemrev.5b00723. - DOI - PMC - PubMed
-
-
- Schuster D. I.Mechanistic Issues in [2 + 2]-Photocycloadditions of Cyclic Enones to Alkenes. In CRC Handbook of Photochemistry and Photobiology, 2nd ed.; Horspool W. M., Lenci F., Eds.; CRC Press: Boca Raton, 2004; pp 72/1–72/24.
-
- Inoue Y., Ed. Chiral Photochemistry, Molecular and Supramolecular Photochemistry, Vol. 11; Dekker: New York, 2004.
- Ramamurthy V., Inoue Y., Eds. Supramolecular Photochemistry; Wiley: Hoboken, 2011.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

