Engineered matrices for skeletal muscle satellite cell engraftment and function
- PMID: 27269735
- PMCID: PMC5136521
- DOI: 10.1016/j.matbio.2016.06.001
Engineered matrices for skeletal muscle satellite cell engraftment and function
Abstract
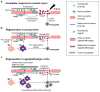
Regeneration of traumatically injured skeletal muscles is severely limited. Moreover, the regenerative capacity of skeletal muscle declines with aging, further exacerbating the problem. Recent evidence supports that delivery of muscle satellite cells to the injured muscles enhances muscle regeneration and reverses features of aging, including reduction in muscle mass and regenerative capacity. However, direct delivery of satellite cells presents a challenge at a translational level due to inflammation and donor cell death, motivating the need to develop engineered matrices for muscle satellite cell delivery. This review will highlight important aspects of satellite cell and their niche biology in the context of muscle regeneration, and examine recent progresses in the development of engineered cell delivery matrices designed for skeletal muscle regeneration. Understanding the interactions of muscle satellite cells and their niche in both native and engineered systems is crucial to developing muscle pathology-specific cell- and biomaterial-based therapies.
Keywords: Aging; Biomaterial; Extracellular matrix; Niche; Satellite cells; Skeletal muscle; Stem cell therapy.
Copyright © 2016 International Society of Matrix Biology. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262–1266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

