Clinical responses with T lymphocytes targeting malignancy-associated κ light chains
- PMID: 27270177
- PMCID: PMC4922690
- DOI: 10.1172/JCI86000
Clinical responses with T lymphocytes targeting malignancy-associated κ light chains
Abstract
Background: Treatment of B cell malignancies with adoptive transfer of T cells with a CD19-specific chimeric antigen receptor (CAR) shows remarkable clinical efficacy. However, long-term persistence of T cells targeting CD19, a pan-B cell marker, also depletes normal B cells and causes severe hypogammaglobulinemia. Here, we developed a strategy to target B cell malignancies more selectively by taking advantage of B cell light Ig chain restriction. We generated a CAR that is specific for the κ light chain (κ.CAR) and therefore recognizes κ-restricted cells and spares the normal B cells expressing the nontargeted λ light chain, thus potentially minimizing humoral immunity impairment.
Methods: We conducted a phase 1 clinical trial and treated 16 patients with relapsed or refractory κ+ non-Hodgkin lymphoma/chronic lymphocytic leukemia (NHL/CLL) or multiple myeloma (MM) with autologous T cells genetically modified to express κ.CAR (κ.CARTs). Other treatments were discontinued in 11 of the 16 patients at least 4 weeks prior to T cell infusion. Six patients without lymphopenia received 12.5 mg/kg cyclophosphamide 4 days before κ.CART infusion (0.2 × 108 to 2 × 108 κ.CARTs/m2). No other lymphodepletion was used.
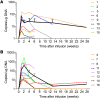
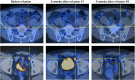
Results: κ.CART expansion peaked 1-2 weeks after infusion, and cells remained detectable for more than 6 weeks. Of 9 patients with relapsed NHL or CLL, 2 entered complete remission after 2 and 3 infusions of κ.CARTs, and 1 had a partial response. Of 7 patients with MM, 4 had stable disease lasting 2-17 months. No toxicities attributable to κ.CARTs were observed.
Conclusion: κ.CART infusion is feasible and safe and can lead to complete clinical responses.
Trial registration: ClinicalTrials.gov NCT00881920.
Funding: National Cancer Institute (NCI) grants 3P50CA126752 and 5P30CA125123 and Leukemia and Lymphoma Society (LLS) Specialized Centers of Research (SCOR) grant 7018.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

