Denosumab or Zoledronic Acid in Postmenopausal Women With Osteoporosis Previously Treated With Oral Bisphosphonates
- PMID: 27270237
- PMCID: PMC4971333
- DOI: 10.1210/jc.2016-1801
Denosumab or Zoledronic Acid in Postmenopausal Women With Osteoporosis Previously Treated With Oral Bisphosphonates
Abstract
Context: Denosumab and zoledronic acid (ZOL) are parenteral treatments for patients with osteoporosis.
Objective: The objective of the study was to compare the effect of transitioning from oral bisphosphonates to denosumab or ZOL on bone mineral density (BMD) and bone turnover.
Design and setting: This was an international, multicenter, randomized, double-blind trial.
Participants: A total of 643 postmenopausal women with osteoporosis previously treated with oral bisphosphonates participated in the study.
Interventions: Subjects were randomized 1:1 to sc denosumab 60 mg every 6 months plus iv placebo once or ZOL 5 mg iv once plus sc placebo every 6 months for 12 months.
Main outcome measures: Changes in BMD and bone turnover markers were measured.
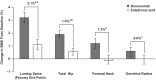
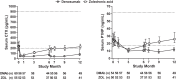
Results: BMD change from baseline at month 12 was significantly greater with denosumab compared with ZOL at the lumbar spine (primary end point; 3.2% vs 1.1%; P < .0001), total hip (1.9% vs 0.6%; P < .0001), femoral neck (1.2% vs -0.1%; P < .0001), and one-third radius (0.6% vs 0.0%; P < .05). The median decrease from baseline was greater with denosumab than ZOL for serum C-telopeptide of type 1 collagen at all time points after day 10 and for serum procollagen type 1 N-terminal propeptide at month 1 and at all time points after month 3 (all P < .05). Median percentage changes from baseline in serum intact PTH were significantly greater at months 3 and 9 with denosumab compared with ZOL (all P < .05). Adverse events were similar between groups. Three events consistent with the definition of atypical femoral fracture were observed (two denosumab and one ZOL).
Conclusions: In postmenopausal women with osteoporosis previously treated with oral bisphosphonates, denosumab was associated with greater BMD increases at all measured skeletal sites and greater inhibition of bone remodeling compared with ZOL.
Trial registration: ClinicalTrials.gov NCT01732770.
Figures

Comment in
-
Letter to the Editor: Bone Turnover as a Potential Determinant of Bone Mineral Density Increase Following the Transition From Bisphosphonates to Either Denosumab or Zoledronic Acid.J Clin Endocrinol Metab. 2016 Sep;101(9):L89-90. doi: 10.1210/jc.2016-2457. J Clin Endocrinol Metab. 2016. PMID: 27600205 No abstract available.
-
Response to the Letter: Bone Turnover as a Potential Determinant of Bone Mineral Density Increase Following the Transition From Bisphosphonates to Either Denosumab or Zoledronic Acid.J Clin Endocrinol Metab. 2016 Sep;101(9):L91-2. doi: 10.1210/jc.2016-2902. J Clin Endocrinol Metab. 2016. PMID: 27600206 No abstract available.
References
-
- Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. - PubMed
-
- Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80:856–861. - PubMed
-
- Gallagher AM, Rietbrock S, Olson M, van Staa TP. Fracture outcomes related to persistence and compliance with oral bisphosphonates. J Bone Miner Res. 2008;23:1569–1575. - PubMed
-
- Gold DT, Martin BC, Frytak JR, Amonkar MM, Cosman F. A claims database analysis of persistence with alendronate therapy and fracture risk in post-menopausal women with osteoporosis. Curr Med Res Opin. 2007;23:585–594. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

