Long-lived antigen-induced IgM plasma cells demonstrate somatic mutations and contribute to long-term protection
- PMID: 27270306
- PMCID: PMC4899631
- DOI: 10.1038/ncomms11826
Long-lived antigen-induced IgM plasma cells demonstrate somatic mutations and contribute to long-term protection
Erratum in
-
Corrigendum: Long-lived antigen-induced IgM plasma cells demonstrate somatic mutations and contribute to long-term protection.Nat Commun. 2016 Aug 16;7:12687. doi: 10.1038/ncomms12687. Nat Commun. 2016. PMID: 27527920 Free PMC article. No abstract available.
Abstract
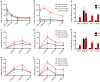
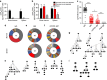
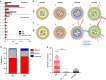
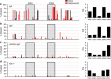
Long-lived plasma cells are critical to humoral immunity as a lifelong source of protective antibodies. Antigen-activated B cells-with T-cell help-undergo affinity maturation within germinal centres and persist as long-lived IgG plasma cells in the bone marrow. Here we show that antigen-specific, induced IgM plasma cells also persist for a lifetime. Unlike long-lived IgG plasma cells, which develop in germinal centres and then home to the bone marrow, IgM plasma cells are primarily retained within the spleen and can develop even in the absence of germinal centres. Interestingly, their expressed IgV loci exhibit somatic mutations introduced by the activation-induced cytidine deaminase (AID). However, these IgM plasma cells are probably not antigen-selected, as replacement mutations are spread through the variable segment and not enriched within the CDRs. Finally, antibodies from long-lived IgM plasma cells provide protective host immunity against a lethal virus challenge.
Figures



References
-
- Slifka M. K., Antia R., Whitmire J. K. & Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity 8, 363–372 (1998). - PubMed
-
- Hammarlund E. et al.. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9, 1131–1137 (2003). - PubMed
-
- Ho F., Lortan J. E., MacLennan I. C. & Khan M. Distinct short-lived and long-lived antibody-producing cell populations. Eur. J. Immunol. 16, 1297–1301 (1986). - PubMed
-
- Di Noia J. M. & Neuberger M. S. Molecular mechanisms of antibody somatic hypermutation. Annu. Rev. Biochem. 76, 1–22 (2007). - PubMed
-
- Nutt S. L., Hodgkin P. D., Tarlinton D. M. & Corcoran L. M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 15, 160–171 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

