Control of the innate immune response by the mevalonate pathway
- PMID: 27270400
- PMCID: PMC4955724
- DOI: 10.1038/ni.3487
Control of the innate immune response by the mevalonate pathway
Abstract
Deficiency in mevalonate kinase (MVK) causes systemic inflammation. However, the molecular mechanisms linking the mevalonate pathway to inflammation remain obscure. Geranylgeranyl pyrophosphate, a non-sterol intermediate of the mevalonate pathway, is the substrate for protein geranylgeranylation, a protein post-translational modification that is catalyzed by protein geranylgeranyl transferase I (GGTase I). Pyrin is an innate immune sensor that forms an active inflammasome in response to bacterial toxins. Mutations in MEFV (encoding human PYRIN) result in autoinflammatory familial Mediterranean fever syndrome. We found that protein geranylgeranylation enabled Toll-like receptor (TLR)-induced activation of phosphatidylinositol-3-OH kinase (PI(3)K) by promoting the interaction between the small GTPase Kras and the PI(3)K catalytic subunit p110δ. Macrophages that were deficient in GGTase I or p110δ exhibited constitutive release of interleukin 1β that was dependent on MEFV but independent of the NLRP3, AIM2 and NLRC4 inflammasomes. In the absence of protein geranylgeranylation, compromised PI(3)K activity allows an unchecked TLR-induced inflammatory responses and constitutive activation of the Pyrin inflammasome.
Conflict of interest statement
The authors declare no competing interests.
Figures
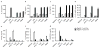


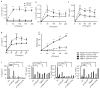



Comment in
-
A dRAStic RHOAdblock of Pyrin inflammasome activation.Nat Immunol. 2016 Jul 19;17(8):900-2. doi: 10.1038/ni.3511. Nat Immunol. 2016. PMID: 27434003 Free PMC article.
References
-
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. - PubMed
-
- Frenkel J, et al. Mevalonate kinase deficiency and Dutch type periodic fever. Clin Exp Rheumatol. 2000;18:525–532. - PubMed
-
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

