Metabolic rewiring in melanoma
- PMID: 27270434
- PMCID: PMC5140782
- DOI: 10.1038/onc.2016.198
Metabolic rewiring in melanoma
Abstract
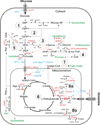
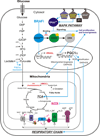
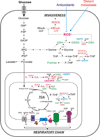
Oncogene-driven metabolic rewiring is an adaptation to low nutrient and oxygen conditions in the tumor microenvironment that enables cancer cells of diverse origin to hyperproliferate. Aerobic glycolysis and enhanced reliance on glutamine utilization are prime examples of such rewiring. However, tissue of origin as well as specific genetic and epigenetic changes determines gene expression profiles underlying these metabolic alterations in specific cancers. In melanoma, activation of the mitogen-activated protein kinase (MAPK) pathway driven by mutant BRAF or NRAS is a primary cause of malignant transformation. Activity of the MAPK pathway, as well as other factors, such as HIF1α, Myc and MITF, are among those that control the balance between non-oxidative and oxidative branches of central carbon metabolism. Here, we discuss the nature of metabolic alterations that underlie melanoma development and affect its response to therapy.
Figures
References
-
- The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. - PubMed
-
- Abildgaard C, Guldberg P. Molecular drivers of cellular metabolic reprogramming in melanoma. Trends Mol Med. 2015;21:164–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

