Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales
- PMID: 27270557
- PMCID: PMC4899628
- DOI: 10.1038/ncomms11833
Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales
Abstract
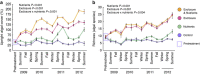
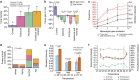
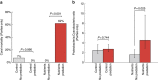
Losses of corals worldwide emphasize the need to understand what drives reef decline. Stressors such as overfishing and nutrient pollution may reduce resilience of coral reefs by increasing coral-algal competition and reducing coral recruitment, growth and survivorship. Such effects may themselves develop via several mechanisms, including disruption of coral microbiomes. Here we report the results of a 3-year field experiment simulating overfishing and nutrient pollution. These stressors increase turf and macroalgal cover, destabilizing microbiomes, elevating putative pathogen loads, increasing disease more than twofold and increasing mortality up to eightfold. Above-average temperatures exacerbate these effects, further disrupting microbiomes of unhealthy corals and concentrating 80% of mortality in the warmest seasons. Surprisingly, nutrients also increase bacterial opportunism and mortality in corals bitten by parrotfish, turning normal trophic interactions deadly for corals. Thus, overfishing and nutrient pollution impact reefs down to microbial scales, killing corals by sensitizing them to predation, above-average temperatures and bacterial opportunism.
Figures

References
-
- Jackson J., Donovan M. K., Cramer K. L. & Lam V. V. Status and Trends of Caribbean Coral Reefs: 1970–2012 Global Coral Reef Monitoring Network, IUCN, Gland (2014).
-
- Wilkinson C. Status of Coral Reefs of the World: 2008 (Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre (2008).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

