Combination inhaled steroid and long-acting beta₂-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease
- PMID: 27271056
- PMCID: PMC6481546
- DOI: 10.1002/14651858.CD008532.pub3
Combination inhaled steroid and long-acting beta₂-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease
Abstract
Background: The long-acting bronchodilator tiotropium and single-inhaler combination therapy of inhaled corticosteroids and long-acting beta2-agonists (ICS/LABA) are commonly used for maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). Combining these treatments, which have different mechanisms of action, may be more effective than administering the individual components.
Objectives: To assess relative effects of the following treatments on markers of exacerbations, symptoms, quality of life and lung function in patients with COPD.• Tiotropium plus LABA/ICS versus tiotropium.• Tiotropium plus LABA/ICS versus LABA/ICS.
Search methods: We searched the Cochrane Airways Group Specialised Register of Trials (April 2015), ClinicalTrials.gov (www.ClinicalTrials.gov), the World Health Organization (WHO) trials portal and reference lists of relevant articles.
Selection criteria: We included parallel, randomised controlled trials (RCTs) lasting three months or longer conducted to compare ICS and LABA combination therapy in addition to inhaled tiotropium versus tiotropium alone or combination therapy alone.
Data collection and analysis: We independently assessed trials for inclusion, then extracted data on trial quality and outcome results. We contacted study authors to ask for additional information. We collected trial information on adverse effects.
Main results: Tiotropium plus LABA/ICS versus tiotropiumWe included six studies (1902 participants) with low risk of bias that compared tiotropium in addition to inhaled corticosteroid and long-acting beta2-agonist combination therapy versus tiotropium alone. Investigators found no statistically significant differences in mortality between treatments (odds ratio (OR) 1.80, 95% confidence interval (CI) 0.55 to 5.91; two studies; 961 participants), a reduction in all-cause hospitalisations with the use of combined therapy (tiotropium + LABA/ICS) (OR 0.61, 95% CI 0.40 to 0.92; two studies; 961 participants; number needed to treat for an additional beneficial outcome (NNTB) 19.7, 95% CI 10.75 to 123.41). The effect on exacerbations was heterogeneous among trials and was not meta-analysed. Health-related quality of life measured by St. George's Respiratory Questionnaire (SGRQ) showed a statistically significant improvement in total scores with use of tiotropium + LABA/ICS compared with tiotropium alone (mean difference (MD) -3.46, 95% CI -5.05 to -1.87; four studies; 1446 participants). Lung function was significantly different in the combined therapy (tiotropium + LABA/ICS) group, although average benefit with this therapy was small. None of the included studies included exercise tolerance as an outcome.A pooled estimate of these studies did not show a statistically significant difference in adverse events (OR 1.16, 95% CI 0.92 to 1.47; four studies; 1363 participants), serious adverse events (OR 0.86, 95% CI 0.57 to 1.30; four studies; 1758 participants) and pneumonia (Peto OR 1.62, 95% CI 0.54 to 4.82; four studies; 1758 participants). Tiotropium plus LABA/ICS versus LABA/ICSOne of the six studies (60 participants) also compared combined therapy (tiotropium + LABA/ICS) versus LABA/ICS therapy alone. This study was affected by lack of power; therefore results did not allow us to draw conclusions for this comparison.
Authors' conclusions: In this update, we found new moderate-quality evidence that combined tiotropium + LABA/ICS therapy compared with tiotropium plus placebo decreases hospital admission. Low-quality evidence suggests an improvement in disease-specific quality of life with combined therapy. However, evidence is insufficient to support the benefit of tiotropium + LABA/ICS for mortality and exacerbations (moderate- and low-quality evidence, respectively). Of note, not all participants enrolled in the included studies would be candidates for triple therapy according to current international guidance.Compared with the use of tiotropium plus placebo, tiotropium + LABA/ICS-based therapy does not increase undesirable effects such as adverse events or serious non-fatal adverse events.
Conflict of interest statement
Dr. OM Garcia has received financial support to attend scientific meetings from pharmaceutical companies which manufacture tiotropium preparations. The remaining three authors were not aware of any conflict of interest that should be declared covering the past three years.
Figures
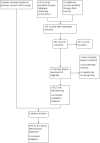
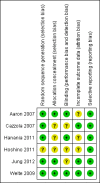

















Update of
-
Combination inhaled steroid and long-acting beta(2)-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2011 Mar 16;(3):CD008532. doi: 10.1002/14651858.CD008532.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2016 Jun 06;(6):CD008532. doi: 10.1002/14651858.CD008532.pub3. PMID: 21412920 Free PMC article. Updated.
References
References to studies included in this review
Aaron 2007 {published data only}
-
- Aaron SD, Vandemheen K, Ferguson D, FitzGerald M, Maltais F, Boureau J, et al. The Canadian optimal therapy of COPD trial: design, organization and patient recruitment. Canadian Respiratory Journal 2004;11(8):581‐5. - PubMed
-
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone‐salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2007;146(8):545‐55. - PubMed
-
- Najafzadeh M, Marra CA, Sadatsafavi M, Aaron SD, Sullivan SD, Vandemheen KL, et al. Cost effectiveness of therapy with combinations of long acting bronchodilators and inhaled steroids for treatment of COPD. Thorax 2008;63(11):962‐7. - PubMed
-
- Roisman G. Tiotropium in combination with placebo, salmeterol, or fluticasone‐salmeterol for treatment of chronic obstructive pulmonary disease. A randomized trial. Revue de Pneumologie Clinique 2007;63(6):390‐1. - PubMed
Cazzola 2007 {published data only}
-
- Cazzola M, Ando F, Santus P, Ruggeri P, Marco F, Sanduzzi A, et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe‐to‐very severe COPD. Pulmonary Pharmacology and Therapeutics 2007;20(5):556‐61. - PubMed
-
- D'Amato M, Ando F, Santus P, Ruggeri P, Marco F, Cazzola M. Clinical effects of adding fluticasone propionate/salmeterol (FSC) and tiotropium (TIO) in severe‐to‐very severe COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):218.
Hanania 2011 {published data only}
-
- Hanania NA, Crater GD, Morris AN, Emmett AH, O'Dell DM, Niewoehner DE. Benefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPD. Respiratory Medicine 2012;106(1):91‐101. [PUBMED: PMID: 22040533.] - PubMed
Hoshino 2011 {published data only}
Jung 2012 {published data only}
-
- Jung KS, Park HY, Park SY, Kim SK, Kim YK, Shim JJ, et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respiratory Medicine 2012;106(3):382‐9. [MEDLINE: ] - PubMed
Welte 2009 {published data only}
-
- Mittmann N, Hernandez P. Cost effectiveness of budesonide/formoterol added to tiotropium bromide versus placebo added to tiotropium bromide in patients with chronic obstructive pulmonary disease: Australian, Canadian and Swedish Healthcare Perspectives. Pharmacoeconomics 2011;29(5):403‐14. [PUBMED: 21504240] - PubMed
-
- Nielsen R, Kankaanranta H, Bjermer L. Cost effectiveness of adding budesonide/formoterol to tiotropium in COPD in four Nordic countries. Respiratory Medicine 2013;107(11):1709‐21. [DOI: ] - PubMed
-
- Welte T, Hartman L, Polanowski T, Hernandez P. Budesonide/formoterol added to tiotropium is well tolerated and reduces risk of severe exacerbations in COPD patients [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20 San Diego. 2009:A6188 [Poster #215].
-
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Addition of budesonide/formoterol to tiotropium reduces the number of exacerbation days compared with tiotropium alone. Chest 2009;136(4):26S‐f.
-
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Budesonide/formoterol added to tiotropium provides rapid improvements in lung function and ability to undertake morning activities. Chest 2009;136(4):24S‐g.
References to studies excluded from this review
Ando 2008 {published data only}
-
- Ando F, Ruggeri P, Girbino G, Cazzola M. Tiotropium and salmeterol/fluticasone combination do not cause oxygen desaturation in COPD. Respiratory Medicine 2008;102(6):815‐8. - PubMed
Bateman 2008 {published data only}
-
- Bateman E, Van‐Dyk M, Chang A. A pilot study comparing tiotropium to salmeterol plus fluticasone in moderate COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 851.
-
- Bateman ED, Dyk M, Sagriotis A. Comparable spirometric efficacy of tiotropium compared with salmeterol plus fluticasone in patients with COPD: a pilot study. Pulmonary Pharmacology and Therapeutics 2008; Vol. 21, issue 1:20‐5. - PubMed
Biscione 2009 {published data only}
-
- Biscione G, Crigna G, Auciello L, Pasqua F, Cazzola M. Addition of tiotropium (T) to a regular treatment with long‐acting beta‐agonist + inhaled corticosteroid (LABA + ICS) in patients with severe to very‐severe COPD under in‐patient pulmonary rehabilitation program (PRP) [Abstract]. European Respiratory Society Annual Congress; 2009 Sep 12‐16; Vienna. 2009:[P526].
Golabi 2006 {published data only}
-
- Golabi P, Topaloglu N, Karakurt S, Celikel T. Effects of tiotropium and salmeterol/fluticasone combination on lung hyperinflation dyspnea and exercise tolerance in COPD [Abstract]. European Respiratory Journal 2006; Vol. 28, issue Suppl 50:33s [E304].
Hara 2007 {published data only}
-
- Hara K, Kurashima K, Tokunaga D, Ueno M, Aoyagi K, Isobe Z, et al. Single blind comparison of tiotropium and salmeterol plus fluticasone propionate of treatment in patients with chronic obstructive pulmonary disease (COPD) [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:Poster #A1.
Maltais 2013 {published data only}
Perng 2006 {published data only}
-
- Perng DW, Wu CC, Su KC, Lee YC, Perng RP, Tao CW. Additive benefits of tiotropium in COPD patients treated with long‐acting beta2 agonists and corticosteroids. Respirology 2006;11(5):598‐602. - PubMed
Petroianni 2008 {published data only}
-
- Petroianni A, Ceccarelli D, Conti V, Graziani E, Terzano C. Evening administration of tiotropium during combination therapy reduces night symptoms in COPD patients [Abstract]. European Respiratory Society 18th Annual Congress; 2008 Oct 3‐7; Berlin 2008:[E4282].
Sarac 2013 {published data only}
-
- Sarac P. Comparison of the efficacy and safety of long‐acting anticholinergic and a combination of inhaled steroids and long‐acting beta‐2 agonist in moderate chronic obstructive pulmonary disease. European Respiratory Journal 2013;42(Suppl 57):P4143.
Singh 2008 {published data only}
-
- Singh D, Brooks J, Hagan G, Cahn A, O'Connor BJ. Superiority of "triple" therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax 2008;63(7):592‐8. - PubMed
-
- Singh D, Hagan G, Cahn A, Leonard TB, Riley JH, O'Connor BJ. Individual and combined responses to salmeterol/fluticasone propionate combination (SFC) and tiotropium (Tio) shown in a COPD clinical trial [Abstract]. American Thoracic Society International Conference; 2008 May 16‐21; Toronto. 2008:A648[#F10].
Tashkin 2008 {published data only}
-
- Tashkin D. Impact of tiotropium on the course of moderate‐to‐very severe chronic obstructive pulmonary disease: the UPLIFT® trial. Expert Review of Respiratory Medicine 2010;4(3):279–89. - PubMed
Troosters 2008 {published data only}
-
- Troosters T, Celli B, Kesten S. Effectiveness of combination therapy with tiotropium in COPD. A secondary analysis of the UPLIFT trial. Primary Care Respiratory Journal 2010;19(2):A13.
References to studies awaiting assessment
Fang 2008 {published data only}
-
- Fang L, Liang X, Zhang F, Liu L, Fu W, Zhao Z, et al. Combination of inhaled salmeterol/fluticasone and tiotropium in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Zhonghua Jiehe He Huxi Zazhi [Chinese Journal of Tuberculosis and Respiratory Diseases] 2008;31(11):811‐4. - PubMed
References to ongoing studies
Betsuyaku 2013 {published data only}
Cohuet 2013 {published data only}
-
- Cohuet Geraldine. A study to compare the effect of inhaled treatments: the combination of 3 components (beclometasone/formoterol/glycopyrrolate) to a known single treatment (tiotropium) or the double combination of tiotropium (Spiriva) and beclometasone plus formoterol in patients with chronic obstructive pulmonary disease treated for one year.. The European Union Clinical Trials Register 2013‐10‐11. [EudraCT Number:2013‐000063‐91]
Additional references
Appleton 2006
Barr 2005
Beeh 2010
-
- Beeh KM, Beier J. The short, the long and the "ultra‐long": why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Advances in Therapy 2010;27(3):150‐9. - PubMed
Brichetto 2003
-
- Brichetto L, Song P, Crimi E. Modulation of cholinergic responsiveness through the [beta]‐adrenoceptor signal transmission pathway in bovine trachealis. Journal of Applied Physiology 2003;95(2):735‐41. - PubMed
Cazzola 2008
-
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. American Thoracic Society; European Respiratory Society Task Force on outcomes of COPD. Outcomes for COPD pharmacological trials: from lung function to biomarkers. European Respiratory Journal 2008;31(2):416–69. [DOI: 10.1183/09031936.00099306] - DOI - PubMed
Cazzola 2010
Drummond 1996
Effing 2007
GOLD 2015
-
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). http://www.goldcopd.org/ (accessed 11 June 2015).
GRADE 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. http://www.guidelinedevelopment.org/handbook/#h.buaodtl66dyx (accessed 11 June 2015).
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383–94. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org (accessed 11 June 2015).
Hutchinson 2010
-
- Hutchinson A, Brand C, Irving L, Roberts C, Thompson P, Campbell D. Acute care costs of patients admitted for management of chronic obstructive pulmonary disease exacerbations: contribution of disease severity, infection and chronic heart failure. Internal Medical Journal 2010;40(5):364‐71. [1445‐5994: (Electronic)] - PubMed
Jones 2005
-
- Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD 2005;2:75‐9. - PubMed
Karner 2014
Lacasse 2006
Liu 2014
Meguro 2007
-
- Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD‐specific version of the St. George Respiratory Questionnaire. Chest 2007; Vol. 132, issue 2:456‐63. - PubMed
Miravitlles 2004
Mittmann 2011
-
- Mittmann N, Hernandez P. Cost effectiveness of budesonide/formoterol added to tiotropium bromide versus placebo added to tiotropium bromide in patients with chronic obstructive pulmonary disease: Australian, Canadian and Swedish Healthcare Perspectives. Pharmacoeconomics 2011;29(5):403‐14. [PUBMED: 21504240] - PubMed
Najafzadeh 2008
-
- Najafzadeh M, Marra CA, Sadatsafavi M, Aaron SD, Sullivan SD, Vandemheen KL, et al. Cost effectiveness of therapy with combinations of long acting bronchodilators and inhaled steroids for treatment of COPD. Thorax 2008;63(11):962‐7. - PubMed
Nannini 2007a
Nannini 2007b
Nannini 2013
Nielsen 2013
-
- Nielsen R, Kankaanranta H, Bjermer L. Cost effectiveness of adding budesonide/formoterol to tiotropium in COPD in four Nordic countries. Respiratory Medicine 2013;107(11):1709‐21. [DOI: ] - PubMed
RevMan 2014 [Computer program]
-
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan). Version 5.3.5. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Rodrigo 2012
Sehatzadeh 2012
Welsh 2010
Westwood 2011
References to other published versions of this review
Karner 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

