Systematic Population Screening, Using Biomarkers and Genetic Testing, Identifies 2.5% of the U.K. Pediatric Diabetes Population With Monogenic Diabetes
- PMID: 27271189
- PMCID: PMC5018394
- DOI: 10.2337/dc16-0645
Systematic Population Screening, Using Biomarkers and Genetic Testing, Identifies 2.5% of the U.K. Pediatric Diabetes Population With Monogenic Diabetes
Abstract
Objective: Monogenic diabetes is rare but is an important diagnosis in pediatric diabetes clinics. These patients are often not identified as this relies on the recognition of key clinical features by an alert clinician. Biomarkers (islet autoantibodies and C-peptide) can assist in the exclusion of patients with type 1 diabetes and allow systematic testing that does not rely on clinical recognition. Our study aimed to establish the prevalence of monogenic diabetes in U.K. pediatric clinics using a systematic approach of biomarker screening and targeted genetic testing.
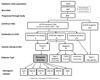
Research design and methods: We studied 808 patients (79.5% of the eligible population) <20 years of age with diabetes who were attending six pediatric clinics in South West England and Tayside, Scotland. Endogenous insulin production was measured using the urinary C-peptide creatinine ratio (UCPCR). C-peptide-positive patients (UCPCR ≥0.2 nmol/mmol) underwent islet autoantibody (GAD and IA2) testing, with patients who were autoantibody negative undergoing genetic testing for all 29 identified causes of monogenic diabetes.
Results: A total of 2.5% of patients (20 of 808 patients) (95% CI 1.6-3.9%) had monogenic diabetes (8 GCK, 5 HNF1A, 4 HNF4A, 1 HNF1B, 1 ABCC8, 1 INSR). The majority (17 of 20 patients) were managed without insulin treatment. A similar proportion of the population had type 2 diabetes (3.3%, 27 of 808 patients).
Conclusions: This large systematic study confirms a prevalence of 2.5% of patients with monogenic diabetes who were <20 years of age in six U.K. clinics. This figure suggests that ∼50% of the estimated 875 U.K. pediatric patients with monogenic diabetes have still not received a genetic diagnosis. This biomarker screening pathway is a practical approach that can be used to identify pediatric patients who are most appropriate for genetic testing.
© 2016 by the American Diabetes Association.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
References
-
- Pihoker C, Gilliam LK, Ellard S, et al. SEARCH for Diabetes in Youth Study Group. Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A and glucokinase: results from the SEARCH for diabetes in youth. J Clin Endocrinol Metab. 2013;98(10):4055–4062. - PMC - PubMed
-
- Irgens HU, Molnes J, Johansson BB, et al. Prevalence of monogenic diabetes in the population based Norwegian childhood diabetes registry. Diabetologia. 2013;56:1512–1519. - PubMed
-
- Lambert AP, Ellard S, Allen LI, et al. Identifying hepatic nuclear factor 1 alpha mutations in children and young adults with a clinical diagnosis of type 1 diabetes. Diabetes Care. 2003;26:333–337. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous