Tofacitinib versus etanercept or placebo in patients with moderate to severe chronic plaque psoriasis: patient-reported outcomes from a Phase 3 study
- PMID: 27271195
- PMCID: PMC5108430
- DOI: 10.1111/jdv.13702
Tofacitinib versus etanercept or placebo in patients with moderate to severe chronic plaque psoriasis: patient-reported outcomes from a Phase 3 study
Abstract
Background: Tofacitinib is an oral Janus kinase inhibitor that is being investigated for psoriasis. Psoriasis impacts on physical and psychological well-being; improvements in health-related quality of life (HRQoL) with etanercept in psoriasis are well documented.
Objective: To evaluate HRQoL with tofacitinib, vs. placebo or etanercept, in the Phase 3, randomized, placebo-controlled, non-inferiority, Oral-treatment Psoriasis Trial (OPT) Compare Study (NCT01241591).
Methods: Adults with moderate to severe chronic plaque psoriasis were randomized 3:3:3:1 to tofacitinib 10 or 5 mg twice daily (BID), etanercept 50 mg twice weekly or placebo, for 12 weeks. Patient-reported outcomes (PROs) included Dermatology Life Quality Index (DLQI), Itch Severity Item and Patient Global Assessment of psoriasis.
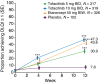
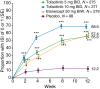
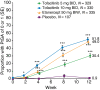
Results: At baseline, 83.4% (911/1092) of patients had a DLQI score ranging between 6 and 30, indicating a substantial burden of disease. By Week 12, 47.3%, 43.6% and 30.9% of patients in the tofacitinib 10 mg BID, etanercept and tofacitinib 5 mg BID groups, respectively, had a DLQI score of 0 or 1 (no effect of psoriasis on QoL) vs. 7.8% for placebo (all P < 0.0001). Tofacitinib significantly reduced itch vs. placebo (P < 0.05 both doses) and etanercept (P < 0.0001 both doses) within 1 day of starting treatment. Furthermore, reductions in itch were greater with tofacitinib 10 mg BID, vs. etanercept, at Weeks 2-12 (all time points P < 0.05). At Week 2, an Itch Severity Item score of 'little or no itch' was more frequent with tofacitinib 10 mg (68.6%) vs. etanercept (57.4%) and placebo (12.2%), and the PtGA response rate was significantly greater with tofacitinib 10 mg vs. placebo (P < 0.05).
Conclusion: Oral tofacitinib provided significant improvements across multiple PROs by Week 12. Improvements with tofacitinib 10 mg BID were comparable to etanercept, and improvements in itch were greater and more rapid with tofacitinib 10 mg BID.
© 2016 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures


References
-
- Gordon KB, Langley RG, Leonardi C et al Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double‐blind, randomized controlled trial and open‐label extension study. J Am Acad Dermatol 2006; 55: 598–606. - PubMed
-
- Reich K, Nestle FO, Papp K et al Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet 2005; 366: 1367–1374. - PubMed
-
- Kimball AB, Papp KA, Wasfi Y et al Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol 2013; 27: 1535–1545. - PubMed
-
- Yosipovitch G, Goon A, Wee J, Chan YH, Goh CL. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol 2000; 143: 969–973. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

