Biologic interventions for fatigue in rheumatoid arthritis
- PMID: 27271314
- PMCID: PMC7175833
- DOI: 10.1002/14651858.CD008334.pub2
Biologic interventions for fatigue in rheumatoid arthritis
Abstract
Background: Fatigue is a common and potentially distressing symptom for patients with rheumatoid arthritis (RA), with no accepted evidence-based management guidelines. Evidence suggests that biologic interventions improve symptoms and signs in RA as well as reducing joint damage.
Objectives: To evaluate the effect of biologic interventions on fatigue in rheumatoid arthritis.
Search methods: We searched the following electronic databases up to 1 April 2014: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Current Controlled Trials Register, the National Research Register Archive, The UKCRN Portfolio Database, AMED, CINAHL, PsycINFO, Social Science Citation Index, Web of Science, and Dissertation Abstracts International. In addition, we checked the reference lists of articles identified for inclusion for additional studies and contacted key authors.
Selection criteria: We included randomised controlled trials if they evaluated a biologic intervention in people with rheumatoid arthritis and had self reported fatigue as an outcome measure.
Data collection and analysis: Two reviewers selected relevant trials, assessed methodological quality and extracted data. Where appropriate, we pooled data in meta-analyses using a random-effects model.
Main results: We identified 32 studies for inclusion in this current review. Twenty studies evaluated five anti-tumour necrosis factor (anti-TNF) biologic agents (adalimumab, certolizumab, etanercept, golimumab and infliximab), and 12 studies focused on five non-anti-TNF biologic agents (abatacept, canakinumab, rituximab, tocilizumab and an anti-interferon gamma monoclonal antibody). All but two of the studies were double-blind randomised placebo-controlled trials. In some trials, patients could receive concomitant disease-modifying anti-rheumatic drugs (DMARDs). These studies added either biologics or placebo to DMARDs. Investigators did not change the dose of the latter from baseline. In total, these studies included 9946 participants in the intervention groups and 4682 participants in the control groups. Overall, quality of randomised controlled trials was moderate with a low to unclear risk of bias in the reporting of the outcome of fatigue. We downgraded the quality of the studies from high to moderate because of potential reporting bias (studies included post hoc analyses favouring reporting of positive result and did not always include all randomised individuals). Some studies recruited only participants with early disease. The studies used five different instruments to assess fatigue in these studies: the Functional Assessment of Chronic Illness Therapy Fatigue Domain (FACIT-F), Short Form-36 Vitality Domain (SF-36 VT), Visual Analogue Scale (VAS) (0 to 100 or 0 to 10) and the Numerical Rating Scale (NRS). We calculated standard mean differences for pooled data in meta-analyses. Overall treatment by biologic agents led to statistically significant reduction in fatigue with a standardised mean difference of -0.43 (95% confidence interval (CI) -0.38 to -0.49). This equates to a difference of 6.45 units (95% CI 5.7 to 7.35) of FACIT-F score (range 0 to 52). Both types of biologic agents achieved a similar level of improvement: for anti-TNF agents, this stood at -0.42 (95% CI -0.35 to -0.49), equivalent to 6.3 units (95% CI 5.3 to 7.4) on the FACIT-F score; and for non-anti-TNF agents, it was -0.46 (95% CI -0.39 to -0.53), equivalent to 6.9 units (95% CI 5.85 to 7.95) on the FACIT-F score. In most studies, the double-blind period was 24 weeks or less. No study assessed long-term changes in fatigue.
Authors' conclusions: Treatment with biologic interventions in patients with active RA can lead to a small to moderate improvement in fatigue. The magnitude of improvement is similar for anti-TNF and non-anti-TNF biologics. However, it is unclear whether the improvement results from a direct action of the biologics on fatigue or indirectly through reduction in inflammation, disease activity or some other mechanism.
Conflict of interest statement
At the time of protocol development Sarah Hewlett was in receipt of a small unrestricted educational grant from GlaxoSmithKline to partially fund a PhD studentship on fatigue measurement in RA and also undertaking an RCT of cognitive behavioural therapy (CBT) for the self‐management of RA fatigue, funded by the Arthritis Research Campaign. During the full review process Sarah Hewlett has been undertaking an RCT of CBT for the self‐management of RA fatigue by the clinical team, funded by the National Institutes for Health Research. She has received small consultancy fees from UCB Pharmaceuticals and Bristol Myers Squibb to advise on the translation of the Bristol RA Fatigue Scales, and small, unrestricted educational grants from Pfizer to deliver training days for staff, in which non‐pharmacological management of fatigue was included. These associations reflect our large programme of research in fatigue into RA but do not constitute a conflict of interests. Robin Christensen has received consulting fees paid to the Parker Institute from Abbott/AbbVie, Axellus A/S, Bristol‐Myers Squibb, Cambridge Weight Plan, Norpharma, Pfizer and Roche; speakers fees paid to the Parker Institute from Axellus A/S, Cambridge Weight Plan, Mundipharma, Roche, and Rottapharm‐MEDA; research grants paid to the Parker Institute from Abbott/AbbVie, Axellus, Bayer HealthCare Pharmaceuticals, Biogen Idec, Bristol‐Myers Squibb, Cambridge Weight Plan, Ipsen, Laboratoires Expanscience, MSD, Mundipharma/Norpharma, Pfizer, and Roche. John Kirwan has been a paid adviser to the Royal Pharmaceutical Society and British Medical Association publication the British National Formulary during the time of this review. He also had unconditional educational grants from Horizon Pharma for the cost of attending the American College of Rheumatology Annual Scientific Meeting in 2013 and the cost of travel to the American College of Rheumatology Annual Scientific Meeting in 2014. Ernest Choy and Cardiff University has received research grants from Ferring Pharmaceuticals, Novimmune, Pfizer, Roche, and UCB. Ernest Choy has received payment as member of advisory boards and lecture fees from Amgen, Biogen, BMS, Celgene, Chugai Pharma, Eli Lilly, Ferring Pharmacuetical, Hospita, Jenssen, MSD, Napp, Novimmune, Pfizer, Regeneron, Roche, Sanofi, Tonix and UCB. Trudie Chalder is author of self help books for chronic fatigue and receives some royalties. Celia Almeida, Fiona Camp and Jon Pollock have no conflict of interest to declare.
Figures
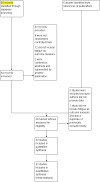

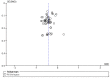
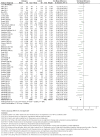
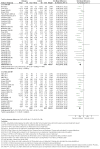
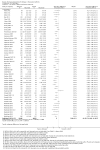
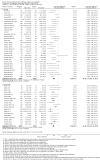
Update of
References
References to studies included in this review
Alten 2011 {published data only}
Bae 2013 {published data only (unpublished sought but not used)}
Choy 2012 {published and unpublished data}
-
- Choy E, McKenna F, Vencovsky J, Valente R, Goel N, Vanlunen B, et al. Certolizumab pegol plus MTX administered every 4 weeks is effective in patients with RA who are partial responders to MTX. Rheumatology 2012;51(7):1226‐34. - PubMed
Cohen 2006 {published data only}
-
- Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti‐tumor necrosis factor therapy: Results of a multicenter, randomized, double‐blind, placebo‐controlled, phase III trial evaluating primary efficacy and safety at twenty‐four weeks. Arthritis and Rheumatism 2006;54(9):2793‐806. - PubMed
-
- Keystone E, Burmester GR, Furie R, Loveless JE, Emery P, Kremer J, et al. Improvement in patient‐reported outcomes in a rituximab trial in patients with severe rheumatoid arthritis refractory to anti‐tumor necrosis factor therapy. Arthritis and Rheumatism 2008;59(6):785‐93. - PubMed
Emery 2006 {published data only}
-
- Emery P, Fleischmann R, Filipowicz‐Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIb randomized, double‐blind, placebo‐controlled, dose‐ranging trial. Arthritis and Rheumatism 2006;54(5):1390‐400. - PubMed
-
- Mease PJ, Revicki DA, Szechinski J, Greenwald M, Kivitz A, Barile‐Fabris L, et al. Improved health‐related quality of life for patients with active rheumatoid arthritis receiving rituximab: results of the Dose‐Ranging Assessment: International Clinical Evaluation of Rituximab in Rheumatoid Arthritis (DANCER) trial. Journal of Rheumatology 2008;35(1):20‐30. - PubMed
Emery 2008 {published data only}
-
- Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double‐blind, parallel treatment trial. The Lancet 2008;372(9636):375‐82. - PubMed
-
- Kekow J, Moots RJ, Emery P, Durez P, Koenig A, Singh A, et al. Patient‐reported outcomes improve with etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial. Annals of the Rheumatic Diseases 2010;69(1):222‐5. - PubMed
Emery 2009 {published data only}
-
- Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, et al. Golimumab, a human anti‐tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate‐naive patients with active rheumatoid arthritis: Twenty‐four‐week results of a phase III, multicenter, randomized, double‐blind, placebo‐controlled study of golimumab before methotrexate as first‐line therapy for early‐onset rheumatoid arthritis. Arthritis and Rheumatism 2009;60(8):2272‐83. - PubMed
Fleischmann 2009 {published data only}
-
- Fleischmann R, Vencovsky J, Vollenhoven R F, Borenstein D, Box J, Coteur G, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease‐modifying antirheumatic therapy: the FAST4WARD study. Annals of the Rheumatic Diseases 2009;68(6):805‐11. - PMC - PubMed
Genovese 2005 {published data only}
-
- Genovese MC, Becker J, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. New England Journal of Medicine 2005;353(11):1114‐23. - PubMed
-
- Genovese MC, Schiff M, Luggen M, Becker JC, Aranda R, Teng J, et al. Efficacy and safety of the selective co‐stimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti‐tumour necrosis factor therapy. Annals of the Rheumatic Diseases 2008;67(4):547‐54. - PubMed
-
- Ostor AJ. Abatacept: a T‐cell co‐stimulation modulator for the treatment of rheumatoid arthritis. Clinical Rheumatology 2008;27(11):1343‐53. Erratum in Clin Rheumatol. 2008 Nov;27(11):1477. - PubMed
-
- Russell A, Dougados M, Li T, Sherrer Y, Becker J, Gandhi M, et al. Abatacept reduces fatigue and pain severity and sleep problems through 2 years in rheumatoid arthritis patients in the AIM and ATTAIN trials. Journal of Rheumatology 2008;35(6):1178.
-
- Russell A, Li T, Genovese M, Pineda C. In rheumatoid arthritis patients with an inadequate response to anti‐TNF therapy in the Attain trial, abatacept effectively reduced pain and fatigue, and improved sleep quality. Journal of Rheumatology 2006;33(2):394.
Genovese 2008 {published data only}
-
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, Silva NA, Alecock E, et al. Interleukin‐6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease‐modifying antirheumatic drugs: the tocilizumab in combination with traditional disease‐modifying antirheumatic drug therapy study. Arthritis and Rheumatism 2008;58(10):2968‐80. - PubMed
Hørslev‐Petersen 2014 {published data only}
-
- Hørslev‐Petersen K, Hetland ML, Junker P, Pødenphant J, Ellingsen T, Ahlquist P, et al. Adalimumab added to a treat‐to‐target strategy with methotrexate and intra‐articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: an investigator‐initiated, randomised, double‐blind, parallel‐group, placebo‐controlled trial. Annals of the Rheumatic Diseases 2014;73(4):654‐61. - PubMed
Keystone 2004 {published data only}
-
- Keystone E, Kavanaugh A, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical and functional outcomes of treatment with adalimumab (a human anti‐tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy. Arthritis and Rheumatism 2004;50(5):1400‐11. - PubMed
Keystone 2009 {published and unpublished data}
-
- Genovese MC, Han C, Keystone EC, Hsia EC, Buchanan J, Gathany T, et al. Effect of golimumab on patient‐reported outcomes in rheumatoid arthritis: results from the GO‐FORWARD study. Journal of Rheumatology 2012;39(6):1185‐91. - PubMed
-
- Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, et al. Golimumab, a human antibody to tumour necrosis factor (alpha) given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO‐FORWARD Study. Annals of the Rheumatic Diseases 2009;68(6):210‐21. - PMC - PubMed
Kremer 2003 {published data only}
-
- Emery P, Kosinski M, Li T, Martin M, Williams GR, Becker JC, et al. Treatment of rheumatoid arthritis patients with abatacept and methotrexate significantly improved health‐related quality of life. Journal of Rheumatology 2006;33(4):681‐9. - PubMed
-
- Kremer JM, Westhovens R, Leon M, Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T‐cell activation with fusion protein CTLA4Ig. New England Journal of Medicine 2003;349(20):1907‐15. - PubMed
Kremer 2006 {published data only}
-
- Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud‐Mendoza C, et al. Effects of abatacept in patients with methotrexate‐resistant active rheumatoid arthritis: a randomized trial. Annals of Internal Medicine 2006;144(12):865‐76. - PubMed
-
- Li T, Gignac M, Wells G, Shen S, Westhovens R, Li T, et al. Decreased external home help use with improved clinical status in rheumatoid arthritis: an exploratory analysis of the Abatacept in Inadequate responders to Methotrexate (AIM) trial. Clinical Therapeutics 2008;30(4):734‐48. - PubMed
-
- Wells G, Li T, Maxwell L, MacLean R, Tugwell P. Determining the minimal clinically important differences in activity, fatigue, and sleep quality in patients with rheumatoid arthritis. Journal of Rheumatology 2007;34(2):280‐9. - PubMed
Li 2013 {published data only (unpublished sought but not used)}
-
- Li Z, Zhang F, Kay J, Fei K, Han C, Zhuang Y, Wu Z. Safety and efficacy of subcutaneous golimumab In Chinese patients with active rheumatoid arthritis despite MTX therapy: results from a randomized, placebo‐controlled, phase 3 trial. Arthritis and Rheumatology 2013;65(Suppl 10):1416. [DOI: 10.1002/art.2013.65.issue-s10] - DOI - PubMed
Lukina 1998 {published data only}
-
- Lukina GV, Sigidin IaA, Skurkovich SV, Skurkovich BS. New approaches to biological immunomodulation therapy of rheumatoid arthritis: neutralization of basic cytokines. Terapevticheskiĭ Arkhiv 1998;70(5):32‐7. Russian. - PubMed
Maini 1999 {published data only}
-
- Maini R, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis and Rheumatism 2004;50(4):1051‐65. - PubMed
-
- Maini R, Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A randomised phase III trial. The Lancet 1999;354(9194):1932‐9. - PubMed
Mittendorf 2007 {published data only}
-
- Mittendorf T, Dietz B, Sterz R, Kupper H, Cifaldi M A, Schulenburg JM. Improvement and longterm maintenance of quality of life during treatment with adalimumab in severe rheumatoid arthritis. Journal of Rheumatology 2007;34(12):2343‐50. - PubMed
-
- Mittendorf T, Sterz R, Schulenburg J, Kupper H, Cifaldi M, Dietz B. Effects of long‐term adalimumab therapy on health utility and fatigue in patients with long‐standing, severe rheumatoid arthritis (RA) ‐ Results from a 3‐year follow‐up study. Value in Health 2005;8(6):A30.
Moreland 1999 {published data only}
-
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. The New England Journal of Medicine 2000;343(22):1586‐93. - PubMed
-
- Moreland LW, Genovese MC, Sato R, Singh A, Moreland LW, Genovese MC, et al. Effect of etanercept on fatigue in patients with recent or established rheumatoid arthritis. Arthritis and Rheumatism 2006;55(2):287‐93. - PubMed
-
- Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Annals of Internal Medicine 1999;130(6):478‐86. - PubMed
-
- Singh G, Singh A, Wanke L, Sato R. Rapid improvement of fatigue in patients on etanercept compared to those on methotrexate: A double‐blind randomized trial in 632 MTX‐naive patients with early, active rheumatoid arthritis. Annals of the Rheumatic Diseases 2003;62(Supplement 1):157.
Pope 2012 {published data only}
-
- Pope J, Fleischmann R, Dougados M, Bingham CO, Massarotti EM, Wollenhaupt J, et al. Rapid reductions in fatigue and sleep problems and correlation with improvements in patient‐reported outcomes in patients with active rheumatoid arthritis treated with certolizumab pegol in the realistic 12‐week phase IIIb randomized controlled study. Rheumatology 2014;53(Supplement 3):43‐5.
Rigby 2011 {published data only}
-
- Rigby W, Ferraccioli G, Greenwald M, Zazueta‐Montiel B, Fleischmann R, Wassenberg S, et al. Effect of rituximab on physical function and quality of life in patients with rheumatoid arthritis previously untreated with methotrexate. Arthritis Care and Research 2011;63(5):711‐20. - PubMed
Schiff 2008 {published data only}
-
- Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi‐centre, randomised, double‐blind, placebo‐controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Annals of the Rheumatic Diseases 2008;67(8):1096‐103. - PMC - PubMed
-
- Vollenhoven R, Dougados M, Keiserman M, Codding C, Songcharoen S, Berman A, et al. Efficacy of abatacept or infliximab in RA patients with an inadequate response to MTX: Results from a 1‐year double‐blind randomized clinical trial. Scandinavian Journal of Rheumatology 2008;37(Supplment 123):51.
Smolen 2008 {published data only}
-
- Smolen JS, Beaulieu A, Rubbert‐Roth A, Ramos‐Remus C, Rovensky J, Alecock E, et al. Effect of interleukin‐6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double‐blind, placebo‐controlled, randomised trial. The Lancet 2008;371(9617):987‐97. - PubMed
Smolen 2009a {published data only}
Smolen 2009b {published data only}
-
- Smolen JS, Kay J, Doyle MK, Landewé R, Matteson EL, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO‐AFTER study): a multicentre, randomised, double‐blind, placebo‐controlled, phase III trial. The Lancet 2009;374(9685):210‐21. - PubMed
Soubrier 2009 {published data only}
-
- Soubrier M, Puéchal X, Sibilia J, Mariette X, Meyer O, Combe B, et al. Evaluation of two strategies (initial methotrexate monotherapy vs its combination with adalimumab) in management of early active rheumatoid arthritis: data from the GUEPARD trial. Rheumatology 2009;48(11):1429‐34. - PubMed
Strand 2009 {published data only}
-
- Keystone E, Heijde D, Mason D, Landewe R, Vollenhoven R, Combe B, et al. Certolizumab Pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis. Arthritis and Rheumatism 2008;58(11):3319‐29. - PubMed
-
- Strand V, Mease P, Burmester GR, Nikai E, Coteur G, Vollenhoven R, et al. Rapid and sustained improvements in health‐related quality of life, fatigue, and other patient‐reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial. Arthritis Research and Therapy 2009;11(6):R170. - PMC - PubMed
Strand 2012a {published data only}
-
- Strand V, Burmester GR, Ogale S, Devenport J, John A, Emery P. Improvements in health‐related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24‐week randomized controlled RADIATE study. Rheumatology (Oxford) 2012;51(10):1860‐9. - PMC - PubMed
Strand 2012b {published data only}
-
- Strand V, Rentz AM, Cifaldi MA, Chen N, Roy S, Revicki D. Health‐related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: results from a randomized multicenter study. Journal of Rheumatology 2012;39(1):63‐72. - PubMed
Weinblatt 2003 {published data only}
-
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis and Rheumatism 2003;48(1):35‐45. Erratum in Arthritis Rheum 2003 Mar;48(3):855. - PubMed
Weinblatt 2013 {published data only}
-
- Weinblatt ME, Bingham CO 3rd, Mendelsohn AM, Kim L, Mack M, Lu J, et al. Intravenous golimumab is effective in patients with active rheumatoid arthritis despite methotrexate therapy with responses as early as week 2: results of the phase 3, randomised, multicentre, double‐blind, placebo‐controlled GO‐FURTHER trial. Annals of the Rheumatic Diseases 2013;72(3):381‐9. - PubMed
References to studies excluded from this review
Breedveld 2005 {published data only}
Cella 2005 {published data only}
-
- Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J, et al. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. Journal of Rheumatology 2005;32(5):811‐9. - PubMed
Dougados 2007 {published data only}
-
- Dougados M, Russell A, Li T, Sherrer Y, Teng J, McCann T, et al. Abatacept provides sustained improvements in pain, fatigue and sleep quality through 2 years in the treatment of rheumatoid arthritis patients in the aim and attain trials. Journal of Rheumatology 2007;34(7):1629.
Duggan 2009 {published data only}
-
- Duggan ST, Keam SJ. Certolizumab pegol in rheumatoid arthritis. BioDrugs 2009;23(6):407‐17. - PubMed
Elliott 1994 {published data only (unpublished sought but not used)}
-
- Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, et al. Randomised double‐blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. The Lancet 1994;344(8930):1105‐10. - PubMed
Frampton 2007 {published data only}
-
- Frampton JE, Scott LJ, Frampton JE, Scott LJ. Rituximab in rheumatoid arthritis. BioDrugs 2007;21(5):333‐41: discussion 342. - PubMed
Furst 2003 {published data only}
-
- Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti tumor necrosis factor‐alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). Journal of Rheumatology 2003;30(12):2563‐71. - PubMed
Genovese 2010 {published data only}
-
- Genovese MC, Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, et al. LY2439821, a humanized anti‐interleukin‐17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double‐blind, placebo‐controlled, proof‐of‐concept study. Arthritis and Rheumatism 2010;62(4):929‐39. - PubMed
Gnanasakthy 2013 {published data only}
-
- Gnanasakthy A, Kosinski M, Genovese M, Mallya U, Mpofu S. Health‐related quality of life (HRQOL) benefits associated with reductions in disease activity and pain severity among rheumatoid arthritis (RA) patients treated with secukinumab. Annals of the Rheumatic Diseases 2012;71(Suppl 3):667.
Grigor 2004 {published data only}
-
- Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single blind randomised controlled trial. The Lancet 2004;364(9430):263‐9. - PubMed
Haugeberg 2009 {published data only}
-
- Haugeberg G, Conaghan PG, Quinn M, Emery P. Bone loss in patients with active early rheumatoid arthritis: infliximab and methotrexate compared with methotrexate treatment alone. Explorative analysis from a 12‐month randomised, double‐blind, placebo‐controlled study. Annals of the Rheumatic Diseases 2009;68(12):1898‐901. - PubMed
Kavanaugh 2003 {published data only}
-
- Kavanaugh A, Genovese M, Baughman J, Kivitz A, Bulpitt K, Olsen N, et al. Allele and antigen‐specific treatment of rheumatoid arthritis: a double blind, placebo controlled phase trial. Journal of Rheumatology 2003;30(3):449‐54. - PubMed
Kavanaugh 2012 {published data only}
-
- Kavanaugh A, Vollenhoven RF, Emery P, et al. Patient‐reported outcomes in early RA patients failing to achieve stable low disease activity: Comparing addition of adalimumab to methotrexate monotherapy with maintenance on adalimumab plus methotrexate.. Arthritis and Rheumatism. 2012; Vol. 64, issue S10:S162‐S3.
Kim 2007 {published data only}
-
- Kim HY, Lee SK, Song YW, Yoo DH, Koh EM, Yoo B, et al. A randomized, double‐blind, placebo‐controlled, phase III study of the human anti‐tumor necrosis factor antibody adalimumab administered as subcutaneous injections in Korean rheumatoid arthritis patients treated with methotrexate. APLAR Journal of Rheumatology 2007;10(1):9‐16.
Kosinski 2000 {published data only}
-
- Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE Jr. Determining minimally important changes in generic and disease‐specific health‐related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis and Rheumatism 2000;43(7):1478‐87. - PubMed
Kremer 2008 {published data only}
-
- Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud‐Mendoza C, et al. Results of a two‐year follow‐up study of patients with rheumatoid arthritis who received a combination of abatacept and methotrexate. Arthritis and Rheumatism 2008;58(4):953‐63. - PubMed
Moreland 2000 {published data only}
-
- Moreland LW, McCabe DP, Caldwell JR, Sack M, Weisman M, Henry G, et al. Phase I/II trial of recombinant methionyl human tumor necrosis factor binding protein PEGylated dimer in patients with active refractory rheumatoid arthritis. Journal of Rheumatology 2000;27(3):601‐9. - PubMed
Moreland 2002 {published data only}
-
- Moreland LW, Alten R, Bosch F, Appelboom T, Leon M, Emery P, et al. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose‐finding, double‐blind, placebo‐controlled clinical trial evaluating CTLA‐4Ig and LEA29Y eighty‐five days after the first infusion. Arthritis and Rheumatism 2002;46(6):1470‐9. - PubMed
Sansonno 2003 {published data only}
-
- Sansonno D, Re V, Lauletta G, Tucci FA, Boiocchi M, Dammacco F. Monoclonal antibody treatment of mixed cryoglobulinemia resistant to interferon alpha with an anti‐CD20. Blood 2003;101(10):3818‐26. - PubMed
Song 2007 {published data only}
-
- Song YW, Lee EY, Koh EM, Cha HS, Yoo B, Lee CK, et al. Assessment of comparative pain relief and tolerability of SKI306X compared with celecoxib in patients with rheumatoid arthritis: a 6‐week, multicenter, randomized, double‐blind, double‐dummy, phase III, noninferiority clinical trial. Clinical Therapeutics 2007;29(5):862‐73. - PubMed
Strand 2012 {published data only}
-
- Strand V, Jones TV, Li W, Koenig AS, Kotak S. Factors that impact work productivity in the preserve trial: A randomized controlled trial of combination etanercept‐methotrexate therapy in patients with moderately active rheumatoid arthritis. Arthritis and Rheumatism 2012;64(S10):S777.
Strand 2014 {published data only}
-
- Strand V, Kosinski M, Gnanasakthy A, Mallya U, Mpofu S. Secukinumab treatment in rheumatoid arthritis is associated with incremental benefit in the clinical outcomes and HRQoL improvements that exceed minimally important thresholds. Health and Quality of Life Outcomes 2014;12(1):31‐40. - PMC - PubMed
Tak 2008 {published data only}
-
- Tak PP, Thurlings RM, Rossier C, Nestorov I, Dimic A, Mircetic V, et al. Atacicept in patients with rheumatoid arthritis: Results of a multicenter, phase Ib, double‐blind, placebo‐controlled, dose‐escalating, single‐ and repeated‐dose study. Arthritis and Rheumatism 2008;58(1):61‐72. - PubMed
Yount 2007 {published data only}
-
- Yount S, Sorensen MV, Cella D, Sengupta N, Grober J, Chartash EK. Adalimumab plus methotrexate or standard therapy is more effective than methotrexate or standard therapies alone in the treatment of fatigue in patients with active, inadequately treated rheumatoid arthritis. Clinical and Experimental Rheumatology 2007;25(6):838‐46. - PubMed
References to ongoing studies
St Clair 2004 {published data only}
-
- Clair EW, Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis and Rheumatism 2004;50(11):3432‐43. - PubMed
Van der Kooij 2009 {published data only}
-
- Kooij SM, Vries‐Bouwstra JK, Goekoop‐Ruiterman YP, Ewals JA, Han KH, Hazes JM, et al. Patient‐reported outcomes in a randomized trial comparing four different treatment strategies in recent‐onset rheumatoid arthritis. Arthritis and Rheumatism 2009;61(1):4‐12. - PubMed
Westhovens 2006 {published data only}
-
- Westhovens R, Cole JC, Li T, Martin M, Maclean R, Lin P, et al. Improved health‐related quality of life for rheumatoid arthritis patients treated with abatacept who have inadequate response to anti‐TNF therapy in a double‐blind, placebo‐controlled, multicentre randomized clinical trial. Rheumatology 2006;45(10):1238‐46. - PubMed
Additional references
Arnett 1988
-
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis & Rheumatism 1988;31(3):315‐24. - PubMed
Blumenauer 2002
Blumenauer 2003
Chauffier 2012
-
- Chauffier K, Salliot C, Berenbaum F, Sellam J. Effect of biotherapies on fatigue in rheumatoid arthritis: a systematic review of the literature and meta‐analysis.. Rheumatology (Oxford) 2012;51(1):60‐8. - PubMed
Chinn 2000
-
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta‐analysis. Statistics in Medicine 2000;19(22):3127‐31. - PubMed
Cohen 1998
-
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum, 1988.
Conaghan 1999
-
- Conaghan PG, Green MJ, Emery P. Established rheumatoid arthritis. Baillières Clinical Rheumatology 1999;13(4):561‐575. - PubMed
Cramp 2013
Department of Health 2006
-
- Department of Health. The Musculoskeletal Services Framework. London: DoH, 2006. [270211]
Heiberg 2008
-
- Heiberg T, Kvien TK, Mowinckel P, Aletaha D, Smolen JS, Hagen KB. Identification of disease activity and health status cut‐off points for the symptom state acceptable to patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 2008;67(7):967‐71.. - PubMed
Hewlett 2005
-
- Hewlett S, Cockshott Z, Byron M, Kitchen K, Tipler S, Pope D, et al. Patients' perceptions of fatigue in RA: Overwhelming, uncontrollable, ignored. Arthritis & Rheumatism (Arthritis Care & Research) 2005;53(5):697‐702. - PubMed
Hewlett 2007
-
- Hewlett S, Hehir M, Kirwan J. Measuring fatigue in rheumatoid arthritis: a systematic review of scales in use. Arthritis & Rheumatism (Arthritis Care & Research) 2007;57(3):429‐39. - PubMed
Hewlett 2008
-
- Hewlett S, Nicklin J, Treharne GJ. Fatigue in musculoskeletal conditions. Reports on the Rheumatic Diseases (Series 6). Topical Reviews 1. Arthritis Research Campaign 2008 Autumn.
Higgins 2003
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Kirwan 2007
-
- Kirwan J, Minnock P, Adebajo A, Bresnihan B, Choy E, Wit M, et al. Patient Perspective Workshop: Fatigue as a recommended patient‐centred outcome measure in rheumatoid arthritis. Journal of Rheumatology 2007;34(5):1174‐7. - PubMed
Luqmani 2006
-
- Luqmani R, Hennell S, Estrach C, Birrell F, Bosworth A, Davenport G, et al. British Society for Rheumatology and British Health Professionals in Rheumatology Guideline for the Management of Rheumatoid Arthritis (the first two years). Rheumatology (Oxford) 2006; Vol. 45, issue 9:1167‐9. - PubMed
Maxwell 2009
Mertens 2009
Navarro‐Sarabia 2005
Pollard 2006
-
- Pollard LC, Choy EH, Gonzalez J, Khoshaba B, Scott DL. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology (Oxford) 2006;45(7):885‐9. - PubMed
Schünemann 2008
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P et al on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008).. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2008.
Singh 2009
Singh 2011
-
- Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, Filippini G, Skoetz N, Francis D, Lopes LC, Guyatt GH, Schmitt J, Mantia L, Weberschock T, Roos JF, Siebert H, Hershan S, Lunn MP, Tugwell P, Buchbinder R. Adverse effects of biologics: a network meta‐analysis and Cochrane overview. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008794.pub2] - DOI - PMC - PubMed
Wells 2007
-
- Wells G, Li T, Maxwell L, MacLean R, Tugwell P. Determining the minimal clinically important differences in activity, fatigue, and sleep quality in patients with rheumatoid arthritis. Journal of Rheumatology 2007;34(2):280‐9. - PubMed
Wolfe 1996
-
- Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. Journal of Rheumatology 1996;23(8):1407‐17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

