Diagnostic Accuracy of Lateral Flow Urine LAM Assay for TB Screening of Adults with Advanced Immunosuppression Attending Routine HIV Care in South Africa
- PMID: 27271432
- PMCID: PMC4896615
- DOI: 10.1371/journal.pone.0156866
Diagnostic Accuracy of Lateral Flow Urine LAM Assay for TB Screening of Adults with Advanced Immunosuppression Attending Routine HIV Care in South Africa
Abstract
Background: We assessed the diagnostic accuracy of Determine TB-LAM (LF-LAM) to screen for tuberculosis among ambulatory adults established in HIV care in South Africa.
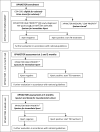
Methods: A systematic sample of adults attending for HIV care, regardless of symptomatology, were enrolled in the XPHACTOR study, which tested a novel algorithm for prioritising investigation with Xpert MTB/RIF. In this substudy, restricted to participants with enrolment CD4<200x106/l, urine was stored at enrolment for later testing with LF-LAM. Sputum was sent for immediate Xpert MTB/RIF if any of: current cough, fever ≥3 weeks, body mass index (BMI)<18.5kg/m2, CD4<100x106/l (or <200x106/l if pre-ART), weight loss ≥10% or strong clinical suspicion were present; otherwise, sputum was stored for Xpert testing at study completion. Participants were reviewed monthly, with reinvestigation if indicated, to 3 months, when sputum and blood were taken for mycobacterial culture. We defined tuberculosis as "confirmed" if Xpert, line probe assay or culture for M. tuberculosis within six months of enrolment were positive, and "clinical" if tuberculosis treatment started without microbiological confirmation.
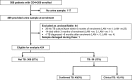
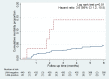
Results: Amongst 424 participants, 61% were female and 57% were taking ART (median duration 22 months); median age, CD4 and BMI were 39 years, 111x106/l, and 23 kg/m2. 56/424 (13%) participants had tuberculosis (40 confirmed, 16 clinical). 24/424 (5.7%) vs. 8/424 (1.9%) were LAM-positive using grade 1 vs. grade 2 cut-off. Using grade 1 cut-off, sensitivity for confirmed TB (all clinical TB excluded) was 12.5% (95% CI 4.2%, 26.8%) and in CD4<100x106/l vs. CD4 ≥100x106/l was 16.7% (95% CI 4.7%, 37.4%) vs. 6.3% (95% CI 0.2%, 30.2%). Specificity was >95% irrespective of diagnostic reference standard, CD4 stratum, or whether grade 1 or grade 2 cut-off was used.
Conclusion: Sensitivity of LF-LAM is too low to recommend as part of intensified case finding in ambulatory patients established in HIV care.
Conflict of interest statement
Figures
References
-
- World Health Organization. Global tuberculosis report 2014 [17th April 2015]. Available: http://www.who.int/tb/publications/global_report/en/.
-
- Samb B, Sow PS, Kony S, Maynart-Badiane M, Diouf G, Cissokho S, et al. Risk factors for negative sputum acid-fast bacilli smears in pulmonary tuberculosis: results from Dakar, Senegal, a city with low HIV seroprevalence. Int J Tuberc Lung Dis. 1999;3(4):330–6. - PubMed
-
- World Health Organization. WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders 2012 [1st May 2013]. Available: http://www.who.int/tb/publications/2012/tb_hiv_policy_9789241503006/en/. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

