Inside out: the role of nucleocytoplasmic transport in ALS and FTLD
- PMID: 27271576
- PMCID: PMC4947127
- DOI: 10.1007/s00401-016-1586-5
Inside out: the role of nucleocytoplasmic transport in ALS and FTLD
Abstract
Neurodegenerative diseases are characterized by the presence of protein inclusions with a different protein content depending on the type of disease. Amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) are no exceptions to this common theme. In most ALS and FTLD cases, the predominant pathological species are RNA-binding proteins. Interestingly, these proteins are both depleted from their normal nuclear localization and aggregated in the cytoplasm. This key pathological feature has suggested a potential dual mechanism with both nuclear loss of function and cytoplasmic gain of function being at play. Yet, why and how this pathological cascade is initiated in most patients, and especially sporadic cases, is currently unresolved. Recent breakthroughs in C9orf72 ALS/FTLD disease models point at a pivotal role for the nuclear transport system in toxicity. To address whether defects in nuclear transport are indeed implicated in the disease, we reviewed two decades of ALS/FTLD literature and combined this with bioinformatic analyses. We find that both RNA-binding proteins and nuclear transport factors are key players in ALS/FTLD pathology. Moreover, our analyses suggest that disturbances in nucleocytoplasmic transport play a crucial initiating role in the disease, by bridging both nuclear loss and cytoplasmic gain of functions. These findings highlight this process as a novel and promising therapeutic target for ALS and FTLD.
Keywords: Aggregation; Exportin; Importin; Neurodegeneration; Nuclear pore; Nuclear transport; Ran-GTP cycle; TDP-43.
Figures

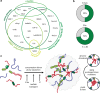

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

