The interplay between effector binding and allostery in an engineered protein switch
- PMID: 27272021
- PMCID: PMC5338240
- DOI: 10.1002/pro.2962
The interplay between effector binding and allostery in an engineered protein switch
Abstract
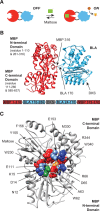
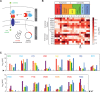
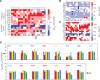
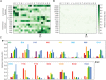
The protein design rules for engineering allosteric regulation are not well understood. A fundamental understanding of the determinants of ligand binding in an allosteric context could facilitate the design and construction of versatile protein switches and biosensors. Here, we conducted extensive in vitro and in vivo characterization of the effects of 285 unique point mutations at 15 residues in the maltose-binding pocket of the maltose-activated β-lactamase MBP317-347. MBP317-347 is an allosteric enzyme formed by the insertion of TEM-1 β-lactamase into the E. coli maltose binding protein (MBP). We find that the maltose-dependent resistance to ampicillin conferred to the cells by the MBP317-347 switch gene (the switch phenotype) is very robust to mutations, with most mutations slightly improving the switch phenotype. We identified 15 mutations that improved switch performance from twofold to 22-fold, primarily by decreasing the catalytic activity in the absence of maltose, perhaps by disrupting interactions that cause a small fraction of MBP in solution to exist in a partially closed state in the absence of maltose. Other notable mutations include K15D and K15H that increased maltose affinity 30-fold and Y155K and Y155R that compromised switching by diminishing the ability of maltose to increase catalytic activity. The data also provided insights into normal MBP physiology, as select mutations at D14, W62, and F156 retained high maltose affinity but abolished the switch's ability to substitute for MBP in the transport of maltose into the cell. The results reveal the complex relationship between ligand binding and allostery in this engineered switch.
Keywords: allostery; ligand binding; maltose binding protein; maltose transport; protein engineering; protein switch.
© 2016 The Protein Society.
Figures

Similar articles
-
Non-allosteric enzyme switches possess larger effector-induced changes in thermodynamic stability than their non-switch analogs.Protein Sci. 2013 Apr;22(4):475-85. doi: 10.1002/pro.2234. Epub 2013 Mar 8. Protein Sci. 2013. PMID: 23400970 Free PMC article.
-
The high-affinity maltose switch MBP317-347 has low affinity for glucose: implications for targeting tumors with metabolically directed enzyme prodrug therapy.Chem Biol Drug Des. 2014 Mar;83(3):266-71. doi: 10.1111/cbdd.12249. Epub 2013 Dec 26. Chem Biol Drug Des. 2014. PMID: 24131788
-
Modular protein switches derived from antibody mimetic proteins.Protein Eng Des Sel. 2016 Feb;29(2):77-85. doi: 10.1093/protein/gzv062. Epub 2015 Dec 5. Protein Eng Des Sel. 2016. PMID: 26637825 Free PMC article.
-
Synthetic protein switches: design principles and applications.Trends Biotechnol. 2015 Feb;33(2):101-10. doi: 10.1016/j.tibtech.2014.11.010. Epub 2014 Dec 20. Trends Biotechnol. 2015. PMID: 25535088 Review.
-
Enhancing the solubility of recombinant proteins in Escherichia coli by using hexahistidine-tagged maltose-binding protein as a fusion partner.Methods Mol Biol. 2011;705:259-74. doi: 10.1007/978-1-61737-967-3_16. Methods Mol Biol. 2011. PMID: 21125392 Review.
Cited by
-
Development and structural characterization of an engineered multi-copper oxidase reporter of protein-protein interactions.J Biol Chem. 2019 Apr 26;294(17):7002-7012. doi: 10.1074/jbc.RA118.007141. Epub 2019 Feb 15. J Biol Chem. 2019. PMID: 30770473 Free PMC article.
-
Converting a Periplasmic Binding Protein into a Synthetic Biosensing Switch through Domain Insertion.Biomed Res Int. 2019 Jan 3;2019:4798793. doi: 10.1155/2019/4798793. eCollection 2019. Biomed Res Int. 2019. PMID: 30719443 Free PMC article. Review.
-
Computational design of Periplasmic binding protein biosensors guided by molecular dynamics.PLoS Comput Biol. 2024 Jun 17;20(6):e1012212. doi: 10.1371/journal.pcbi.1012212. eCollection 2024 Jun. PLoS Comput Biol. 2024. PMID: 38885277 Free PMC article.
-
Full and Partial Agonism of a Designed Enzyme Switch.ACS Synth Biol. 2016 Dec 16;5(12):1475-1484. doi: 10.1021/acssynbio.6b00097. Epub 2016 Jul 22. ACS Synth Biol. 2016. PMID: 27389009 Free PMC article.
-
Expanding the bioanalytical application of β-hydroxybutyrate binding proteins through characterization of their metabolite interactions and site-directed mutagenesis.Protein Sci. 2025 May;34(5):e70129. doi: 10.1002/pro.70129. Protein Sci. 2025. PMID: 40260974
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

