Pharmacokinetics of single dose radium-223 dichloride (BAY 88-8223) in Japanese patients with castration-resistant prostate cancer and bone metastases
- PMID: 27272279
- PMCID: PMC4961730
- DOI: 10.1007/s12149-016-1093-8
Pharmacokinetics of single dose radium-223 dichloride (BAY 88-8223) in Japanese patients with castration-resistant prostate cancer and bone metastases
Abstract
Objective: This open-label, non-randomized, phase I study examined the pharmacokinetics (PK) and radiation dosimetry of a single dose of radium-223 in Japanese patients with castration-resistant prostate cancer (CRPC) and bone metastases.
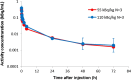
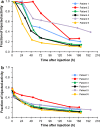
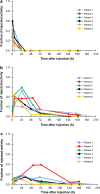
Methods: Six male Japanese patients (mean age 72.5 years, range 65-79 years) with histologically or cytologically confirmed stage IV adenocarcinoma of the prostate were recruited. A single IV dose of radium-223 was delivered intravenously (IV) via slow bolus over a 2-5 min period: Cohort 1 received 50 kBq/kg and Cohort 2 received 100 kBq/kg.
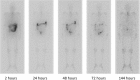
Results: Following IV injection, radium-223 was rapidly eliminated from the blood in a multi-phasic manner. The fraction of the injected activity of radium-223 retained in the whole body 24 h following injection was 85 %. Biodistribution results showed initial bone uptake was 52 % (range 41-57 %). The maximum activity of radium-223 in the bone was observed within 2 h of dosing. Activity of radium-223 passed through the small intestine within 24 h. No activity was detected in other organs. The major radiation dose from radium-223 was found in osteogenic cells; calculated absorbed doses in osteogenic cells and in the red marrow were 0.76 Gy/MBq and 0.09 Gy/MBq, respectively.
Conclusions: In Japanese patients with CRPC and bone metastases, radium-223 (IV) achieved maximum activity in the bone rapidly and passed through the intestine within 24 h, without signs of activity in other organs. The PK profile and absorbed radiation dose in organs and tissues in Japanese patients were similar to data from non-Japanese patients. Trial registration identification: NCT01565746.
Keywords: Castration-resistant prostate cancer; Metastases; Pharmacokinetics; Radium-223.
Figures

References
-
- 1.5-year post-treatment follow-up of radium-223 dichloride (Ra-223) in patients with castration-resistant prostate cancer (CRPC) and bone metastases from the phase 3 ALSYMPCA Study 9. Clin Adv Hematol Oncol. 2014;12(4 Suppl 11):9–10.
-
- Anido Herranz U, Fernandez Calvo O, Afonso Afonso FJ, Rodriguez Martinez de Llano S, Lazaro Quintela M, Leon Mateos L et al. Radium-223 dichloride: a new paradigm in the treatment of prostate cancer. Expert Rev Anticancer Ther. 2015; 1–10. - PubMed
-
- Hoskin P, Sartor O, O’Sullivan JM, Johannessen DC, Helle SI, Logue J, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014;15(12):1397–1406. doi: 10.1016/S1470-2045(14)70474-7. - DOI - PubMed
-
- Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15(7):738–746. doi: 10.1016/S1470-2045(14)70183-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

