Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes
- PMID: 27272351
- PMCID: PMC8563847
- DOI: 10.1002/14651858.CD005542.pub3
Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes
Abstract
Background: Diabetes results in a rise in blood glucose above normal physiological levels; if untreated this may cause damage to many systems including the cardiovascular and renal systems. Pregnancy increases resistance to insulin action; for those women who have pre-gestational diabetes, this results in an increasing insulin requirement. There are several methods of administering insulin. Conventionally, insulin has been administered subcutaneously, formally referred to as intensive conventional treatment, but now more usually referred to as multiple daily injections (MDI). An alternative method of insulin administration is the continuous subcutaneous insulin infusion pump (CSII).
Objectives: To compare CSII with MDI of insulin for pregnant women with pre-existing and gestational diabetes.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 March 2016) and reference lists of retrieved studies.
Selection criteria: Randomised trials comparing CSII with MDI for pregnant women with diabetes.
Data collection and analysis: Three review authors independently assessed studies and two review authors extracted data. Disagreements were resolved through discussion with the third author. We assessed the quality of the evidence using the GRADE approach.
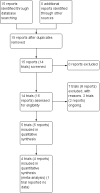
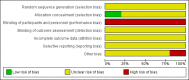
Main results: We included five single-centre trials (undertaken in Italy) with 153 women and 154 pregnancies in this review.There were no clear differences in the primary outcomes reported between CSII and MDI in the included trials: caesarean section (risk ratio (RR) 1.09, 95% confidence interval (CI) 0.66 to 1.77; three trials, 71 women, evidence graded very low), large-for-gestational age (RR 4.15, 95% CI 0.49 to 34.95; three trials, 73 infants; evidence graded very low), and perinatal mortality (RR 2.33, 95% CI 0.38 to 14.32; four trials, 83 infants, evidence graded very low). Other primary outcomes were not reported in these trials (hypertensive disorders of pregnancy, development of type 2 diabetes, composite outcome of serious neonatal outcomes, and neurosensory disability).There was no clear evidence of differences in the maternal secondary outcomes: maternal weight gain during pregnancy, 24 hour mean blood glucose in each trimester, mean maternal HbA1c in each trimester, maternal hypoglycaemia, and maternal hyperglycaemia. The included studies did not report several GRADE outcomes: perineal trauma, return to pre-pregnancy weight, postnatal depression, induction of labour. Many maternal secondary outcomes were also not reported.In two trials, including a total of 61 infants, CSII was associated with an increase in mean birthweight compared with MDI (mean difference (MD) 220.56 g, 95% CI -2.09 g to 443.20 g; P = 0.05). However, the large CI including anything from a small reduction to an increase in mean birthweight and the lack of a difference in macrosomia rate (RR 3.20, CI 0.14 to 72.62; two trials, 61 infants) suggests uncertainty. Large-for-gestational age (see above), andsmall-for-gestational age also suggests uncertainty of effect. No significant differences were found in: gestation at delivery, preterm birth < 37 weeks' gestation, preterm birth < 32 weeks' gestation, neonatal hypoglycaemia (evidence graded very low), respiratory distress syndrome, neonatal hyperbilirubinaemia, and fetal anomaly. There were no data reported on many important infant outcomes, including the GRADE outcomes adiposity and diabetes. There was no follow-up of infants in childhood or adulthood, so longer-term outcomes were not reported.The only outcome reported for use of health service resources wasmaternal days hospitalised, which did not show a difference between groups in the small number of women included (MD 9.40, CI -6.04 to 24.84; one trial, 10 women).The methods used by the trials were poorly reported, for example although blinding of participants and clinicians regarding intervention allocation is impossible, it is possible to blind assessors and this along with other aspects of trial methods was not reported, which means that the trials are at an unclear or high risk of bias. We do not know if the women who participated were representative, and therefore if the results can be generalised. Most GRADE outcomes were not reported. For the GRADE outcomes that were reported, our assessment was that the evidence is very low quality (caesarean section, large-for-gestational age, perinatal mortality, andneonatal hypoglycaemia). This was due to design limitations in the included trials, small sample sizes in the trials contributing data, wide CIs crossing both the line of no effect and the line of appreciable benefit and/or harm, and often few events. We are therefore uncertain whether CSII or MDI improves outcomes for pregnant women with diabetes and their infants, and the results of further studies may differ substantially from those presented in this review.
Authors' conclusions: There is no evidence to support the use of one particular form of insulin administration over another for pregnant women with diabetes. There are only a small number of trials appropriate for meta-analysis, a small number of women included and questionable generalisability of the trial population.Pump technology has progressed since these trials were undertaken. Well-designed randomised trials are required to evaluate comparisons such as patch pumps against MDI and more conventional CSII against MDI. These trials should be adequately powered to assess the effect of interventions, and report the core set of outcomes used in Cochrane reviews of diabetes in pregnancy. Trials to assess the effects of pumps on birthweight and macrosomia rates are needed. It would be beneficial for future trials to undertake longer-term follow-up of participants and their infants, assess women's preferences, and conduct an economic evaluation.
Conflict of interest statement
Helen West is paid to work on Cochrane reviews by a grant to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Figures

























Update of
-
Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes.Cochrane Database Syst Rev. 2007 Jul 18;(3):CD005542. doi: 10.1002/14651858.CD005542.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2016 Jun 07;(6):CD005542. doi: 10.1002/14651858.CD005542.pub3. PMID: 17636806 Updated.
References
References to studies included in this review
Botta 1986 {published data only}
-
- Botta RM, Sinarga D, Angelico MC, Bomplani GD. Intensified conventional insulin therapy as compared to micropump therapy in pregnant women affected by type 1 diabetes mellitus [Confronto fra terapia insulinica tradizionale ottimizzata e con microinfusore in gravide affette da diabete mellito di tipo1]. Minerva Medica 1986;77:657‐61. - PubMed
Carta 1986 {published data only}
-
- Carta Q, Meriggi E, Trossarelli GF, Catella G, Dal Molin V, Menato G, et al. Continuous subcutaneous insulin infusion versus intensive conventional insulin therapy in type I and type II diabetic pregnancy [Etude comparee de la perfusion sous‐cutanee continue d'insuline et de l'insulinotherapie intensive classique chez des femmes diabetiques de type I ou II enceintes]. Diabete et Metabolisme 1986;12:121‐9. - PubMed
Mello 2005 {published data only}
-
- Mello G, Parretti E, Tondi F, Riviello C, Borri P, Scarselli G. Impact of two treatment regimens with insulin lispro in post‐prandial glucose excursion patterns and fetal fat mass growth in type 1 diabetic pregnant women [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S36.
Nosari 1993 {published data only}
-
- Nosari I, Maglio ML, Lepore G, Cortinovis F, Pagani G. Is continuous subcutaneous insulin infusion more effective than intensive conventional insulin therapy in the treatment of pregnant diabetic women?. Diabetes, Nutrition & Metabolism ‐ Clinical & Experimental 1993;6:33‐7.
Trossarelli 1984 {published data only}
-
- Trossarelli GF, Cavallo‐Perin P, Meriggi E, Menato G, Dolfin G, Carta Q, et al. Metabolic and obstetrical results in Type 1 (insulin‐dependent) diabetic pregnancy: pump versus optimized conventional insulin therapy [abstract]. Diabetologia 1984;27(2):340A.
References to studies excluded from this review
Burkart 1988 {published data only}
-
- Burkart W, Hanker JP, Schneider HPG. Complications and fetal outcome in diabetic pregnancy ‐ Intensified conventional verses insulin pump therapy. Gynecologic and Obstetric Investigation 1988;26:104‐12. - PubMed
Collaborative 1993 {published data only}
-
- Collaborative. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. New England Journal of Medicine 1993;329(14):977‐86. - PubMed
Coustan 1986 {published data only}
-
- Coustan DR, Reece A, Sherwin RS, Rudolf MCJ, Bates SE, Sockin SM, et al. A randomised clinical trial of the insulin pump vs intensive conventional therapy in diabetic pregnancy. JAMA 1986;255:631‐6. - PubMed
Ignatova 2007 {published data only}
-
- Ignatova N, Arbatskaya N, Melnikova E. Continuous subcutaneous insulin infusion (CSII) reduces the rate of hypoglycaemic episodes throughout pregnancy. Diabetologia 2007;50(Suppl 1):S383‐4.
Laatikainen 1987 {published data only}
-
- Laatikainen L, Teramo K, Hieta‐Heikuraninen H, Koivisto V, Pelkonen R. A controlled study of the influence of continuous subcutaneous insulin infusion treatment on diabetic retinopathy during pregnancy. Acta Medica Scandinavica 1987;221:367‐76. - PubMed
Murphy 2011 {published data only}
-
- Murphy HR. Evaluation of the safety and efficacy of closed loop glucose control during the activities of normal daily living in women with type 1 diabetes during pregnancy: an open label randomised cross‐over study. ISRCTN Registry (http://www.isrctn.com/) [accessed 2 November 2015] 2010.
-
- Murphy HR, Kumareswaran K, Elleri D, Allen JM, Caldwell K, Biagioni M, et al. Safety and efficacy of 24‐h closed‐loop insulin delivery in well‐controlled pregnant women with type 1 diabetes: a randomized crossover case series.[Erratum appears in Diabetes Care. 2012 Jan;35(1):191]. Diabetes Care 2011;34(12):2527‐9. - PMC - PubMed
Zoupas 1991 {published data only}
-
- Zoupas C. Insulin infusion pumps in pregnancy. Personal communication 1991.
References to ongoing studies
Stewart 2014 {published data only}
-
- Stewart Z. Evaluation of the feasibility, utility, safety and efficacy of overnight closed‐loop insulin delivery at home in women with type 1 diabetes during pregnancy. Acronym: CLIP_03. ISRCTN Registry (http://www.isrctn.com/) [accessed 2 November 2015] 2014.
Thompson 2014 {published data only}
-
- Thompson DM. Comparison of continuous subcutaneous insulin infusion (CSII) with multiple daily injections (MDI) for the treatment of pregestational diabetes during pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 1 November 2015] 2014.
Additional references
Alwan 2009
Anderson 1997
-
- Anderson JH, Brundelle RL, Koivisto VA. Reduction of postprandial hyperglycaemia and frequency of hypoglycaemia in IDDM patients on insulin analog treatment. Multicentre insulin lispro study group. Diabetes 1997;46:265‐70. - PubMed
Brink 1986
-
- Brink SJ, Stewart C. Insulin pump treatment in insulin‐dependent diabetes mellitus. JAMA 1986;255(5):617‐21. - PubMed
Brown 2015
Brown 2015a
Brown 2016
Buhling 2004
-
- Buhling KJ, Kurzidim B, Wolhlfarth K, Mahmoudi M, Wascher C, Siebert G, et al. Introductory experience with the continuous glucose monitoring system (CGMS; Medtronic Minimed) in detecting hyperglycaemia by comparing the self monitoring of blood glucose (SMBG) in non‐pregnant women and pregnant women with impaired glucose tolerance and gestational diabetes. Experimental and Clinical Endocrinology & Diabetes 2004;112(10):556‐60. - PubMed
Catalano 2012
CEMACH 2005
-
- CEMACH. Pregnancy in Women with Type 1 and Type 2 Diabetes 2002‐2003. London: CEMACH, 2005.
CMACE 2010
-
- Centre for Maternal and Child Enquiries (CMACE). Perinatal Mortality 2008: United Kingdom. London: CMACE, 2010.
Colquitt 2004
-
- Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N. Clinical and cost effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technology Assessment Programme 2004;8(9):43. - PubMed
Farrar 2015
Gonzalez 2002
-
- Gonzalez JL. Management of diabetes in pregnancy. Clinical Obstetrics and Gynecology 2002;45(1):165‐9. - PubMed
Hadden 1996
HAPO 2008
-
- HAPO study cooperative research group. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine 2008;358:1991‐2002. - PubMed
HAPO 2010
-
- HAPO Study Cooperative Research Group. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG: an international journal of obstetrics and gynaecology 2010;117:575‐84. - PubMed
Hawthorne 1997
Hawthorne 2002
-
- Hawthorne G, Modder J. Maternity services for women with diabetes in the UK. BMJ 2002;19(Suppl 4):50‐5. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirsch 2005
-
- Hirsch IB, Bode BW, Garg S, Lane WS, Sussman A, Hu P, et al. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005;28(3):533‐8. - PubMed
Johansson 2000
-
- Johansson UB, Adamson UC, Lins PE, Wredling RA. Improved blood glucose variability, HbA1C insuman infusat and less insulin requirement in IDDM patients using insulin lispro in CSII. The Swedish Multicentre Lispro Insulin Study. Diabetes and Metabolism 2000;26:192‐6. - PubMed
John 1997
-
- John WG. Glycated haemoglobin analysis. Annals of Clinical Biochemistry 1997;34:17‐31. - PubMed
Kerssen 2004
-
- Kerssen A, De‐Valk HW, Visser GHA. The continuous monitoring system during pregnancy of women with type 1 diabetes mellitus: accuracy assessment. Diabetes Technology & Therapeutics 2004;6(5):645‐51. - PubMed
Kilpatrick 1997
-
- Kilpatrick ES. Problems in the assessment of glycaemic control in diabetes mellitus. Diabetic Medicine 1997;14:819‐31. - PubMed
Kilpatrick 1998
-
- Kilpatrick ES, Maylor PW, Keevil BG. Biological variation of glycated haemoglobin. Diabetes Care 1998;21(2):261‐4. - PubMed
Knight 1985
Knight 2014
-
- Knight MKS, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk JJ (Eds.) on Behalf of MBRRACE‐UK. Saving Lives, Improving Mothers’ Care: Lessons learned to Inform Future Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009‐2012. National Perinatal Epidemiology Unit, 2014.
Maresh 2001
-
- Maresh M. Diabetes in pregnancy. Current Opinion in Obstetrics and Gynecology 2001;13:103‐7. - PubMed
Marshall 2000
-
- Marshall SM, Barth JH. Standardization of HbA1c measurements‐a consensus statement. Diabetic Medicine 2000;17:5‐6. - PubMed
Martis 2016
Mecklenburg 1984
-
- Mecklenburg RS, Benson EA, Benson JW, Fredlund PN, Guinn T, Metz RJ, et al. Acute complications associated with insulin infusion pump therapy. Report of experience with 161 patients. JAMA 1984;252(23):3265‐9. - PubMed
Melamed 2009
-
- Melamed N, Moshe H. Perinatal mortality in pregestational diabetes. International Journal of Gynaecology and Obstetrics 2009;104:S20‐S24. - PubMed
Middleton 2016
Mukhopadhyay 2007
-
- Mukhopadhyay A, Farrell T, Fraser RB, Ola B. Continuous subcutaneous insulin infusion vs intensive conventional insulin therapy in pregnant diabetic women: a systematic review and metaanalysis of randomized, controlled trials. American Journal of Obstetrics and Gynecology 2007;197:447‐56. - PubMed
Penney 2003
-
- Penney GC, Mair G, Pearson DWM. On behalf of the Scottish Diabetes in Pregnancy Group. Outcomes of pregnancies in women with type 1 diabetes in Scotland: a national population‐based study. BJOG: an international journal of obstetrics and gynaecology 2003;110:315‐8. - PubMed
Porter 2004
-
- Porter H, Belfort MA. Evaluation of a new real‐time continuous glucose monitoring system in pregnant women without gestational diabetes: a pilot study. Journal of Perinatal and Neonatal Nursing 2004;18(2):93‐102. - PubMed
Pozzilli 2016
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowan 2008
-
- Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. Metformin versus insulin for the treatment of gestational diabetes. New England Journal of Medicine 2008;358:2003‐15. - PubMed
Siebenhofer 2004
-
- Siebenhofer A, Plank J, Berghold A, Horvath K, Sawicki PT, Beck P, et al. Meta‐analysis of short‐acting insulin analogues in adult patients with type 1 diabetes: continuous subcutaneous insulin infusion versus injection therapy. Diabetologia 2004;47:1895‐905. - PubMed
Siebenhofer 2006
Thabit 2012
Tieu 2010
Williams 2003
-
- Williams J. Overview of the care of pregnant women with pre‐existing diabetes. Journal of Diabetes Nursing 2003;7:12‐6.
Zaccardi 2016
-
- Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90‐year perspective. Postgraduate Medical Journal 2016;92(1084):63‐9. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

