Glucose induced activation of canonical Wnt signaling pathway in hepatocellular carcinoma is regulated by DKK4
- PMID: 27272409
- PMCID: PMC4897783
- DOI: 10.1038/srep27558
Glucose induced activation of canonical Wnt signaling pathway in hepatocellular carcinoma is regulated by DKK4
Abstract
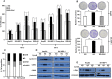
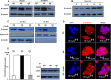
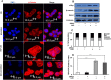
Elevated glycemic index, an important feature of diabetes is implicated in an increased risk of hepatocellular carcinoma (HCC). However, the underlying molecular mechanisms of this association are relatively less explored. Present study investigates the effect of hyperglycemia over HCC proliferation. We observed that high glucose culture condition (HG) specifically activates canonical Wnt signaling in HCC cells, which is mediated by suppression of DKK4 (a Wnt antagonist) expression and enhanced β-catenin level. Functional assays demonstrated that a normoglycemic culture condition (NG) maintains constitutive expression of DKK4, which controls HCC proliferation rate by suppressing canonical Wnt signaling pathway. HG diminishes DKK4 expression leading to loss of check at G0/G1/S phases of the cell cycle thereby enhancing HCC proliferation, in a β-catenin dependent manner. Interestingly, in NOD/SCID mice supplemented with high glucose, HepG2 xenografted tumors grew rapidly in which elevated levels of β-catenin, c-Myc and decreased levels of DKK4 were detected. Knockdown of DKK4 by shRNA promotes proliferation of HCC cells in NG, which is suppressed by treating cells exogenously with recombinant DKK4 protein. Our in vitro and in vivo results indicate an important functional role of DKK4 in glucose facilitated HCC proliferation.
Figures





References
-
- Ferenci P. et al. Hepatocellular carcinoma (HCC): a global perspective. J. Clin. Gastroenterol. 44, 239–245 (2010). - PubMed
-
- Liu C. J. & Kao J. H. Global perspective on the natural history of chronic hepatitis B: role of hepatitis B virus genotypes A to J. Semin. Liver Dis. 33, 92–102 (2013). - PubMed
-
- Venook A. P., Papandreou C., Furuse J. & de Guevara L. L. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 15, 5–13 (2010). - PubMed
-
- Wang P., Kang D., Cao W., Wang Y. & Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab. Res. Rev. 28, 109–122 (2012). - PubMed
-
- Tolman K. G., Fonseca V., Dalpiaz A. & Tan M. H. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 30, 734–743 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

