Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children
- PMID: 27272563
- PMCID: PMC8504985
- DOI: 10.1002/14651858.CD007524.pub4
Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children
Update in
-
Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD007524. doi: 10.1002/14651858.CD007524.pub5. Cochrane Database Syst Rev. 2022. PMID: 36161875 Free PMC article.
Abstract
Background: People with asthma may experience exacerbations or "attacks" during which their symptoms worsen and additional treatment is required. Written action plans may advocate doubling the dose of inhaled steroids in the early stages of an asthma exacerbation to reduce the severity of the attack and to prevent the need for oral steroids or hospital admission.
Objectives: To compare the clinical effectiveness and safety of increased versus stable doses of inhaled corticosteroids (ICS) as part of a patient-initiated action plan for home management of exacerbations in children and adults with persistent asthma.
Search methods: We searched the Cochrane Airways Group Specialised Register, which is derived from searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) to March 2016. We handsearched respiratory journals and meeting abstracts.
Selection criteria: We included randomised controlled trials (RCTs) that compared increased versus stable doses of ICS for home management of asthma exacerbations. We included studies of children or adults with persistent asthma who were receiving daily maintenance ICS.
Data collection and analysis: Two review authors independently selected trials, assessed quality and extracted data. We contacted authors of RCTs for additional information.
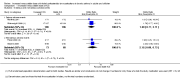
Main results: This review update added three new studies including 419 participants to the review. In total, we identified eight RCTs, most of which were at low risk of bias, involving 1669 participants with mild to moderate asthma. We included three paediatric (n = 422) and five adult (n = 1247) studies; six were parallel-group trials and two had a cross-over design. All but one study followed participants for six months to one year. Allowed maintenance doses of ICS varied in adult and paediatric studies, as did use of concomitant medications and doses of ICS initiated during exacerbations. Investigators gave participants a study inhaler containing additional ICS or placebo to be started as part of an action plan for treatment of exacerbations.The odds of treatment failure, defined as the need for oral corticosteroids, were not significantly reduced among those randomised to increased ICS compared with those taking their usual stable maintenance dose (odds ratio (OR) 0.89, 95% confidence interval (CI) 0.68 to 1.18; participants = 1520; studies = 7). When we analysed only people who actually took their study inhaler for an exacerbation, we found much variation between study results but the evidence did not show a significant benefit of increasing ICS dose (OR 0.84, 95% CI 0.54 to 1.30; participants = 766; studies = 7). The odds of having an unscheduled physician visit (OR 0.96, 95% CI 0.66 to 1.41; participants = 931; studies = 3) or acute visit (Peto OR 0.98, 95% CI 0.24 to 3.98; participants = 450; studies = 3) were not significantly reduced by an increased versus stable dose of ICS, and evidence was insufficient to permit assessment of impact on the duration of exacerbation; our ability to draw conclusions from these outcomes was limited by the number of studies reporting these events and by the number of events included in the analyses. The odds of serious events (OR 1.69, 95% CI 0.77 to 3.71; participants = 394; studies = 2) and non-serious events, such as oral irritation, headaches and changes in appetite (OR 2.15, 95% CI 0.68 to 6.73; participants = 142; studies = 2), were neither increased nor decreased significantly by increased versus stable doses of ICS during an exacerbation. Too few studies are available to allow firm conclusions on the basis of subgroup analyses conducted to investigate the impact of age, time to treatment initiation, doses used, smoking history and the fold increase of ICS on the magnitude of effect; yet, effect size appears similar in children and adults.
Authors' conclusions: Current evidence does not support increasing the dose of ICS as part of a self initiated action plan to treat exacerbations in adults and children with mild to moderate asthma. Increased ICS dose is not associated with a statistically significant reduction in the odds of requiring rescue oral corticosteroids for the exacerbation, or of having adverse events, compared with a stable ICS dose. Wide confidence intervals for several outcomes mean we cannot rule out possible benefits of this approach.
Conflict of interest statement
Kayleigh Kew: none.
Michael Quinn: none.
Bradley Quon: none.
Francine Ducharme: grant support for investigator‐initiated studies from Merck and Co., unrestricted donations from Merck, GlaxoSmithKline, and Takeda, to support an electronic database of children consulting for asthma; member of the advisory boards of Boehringer Ingelheim.
Figures













Update of
-
Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children.Cochrane Database Syst Rev. 2010 Dec 8;(12):CD007524. doi: 10.1002/14651858.CD007524.pub3. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2016 Jun 07;(6):CD007524. doi: 10.1002/14651858.CD007524.pub4. PMID: 21154378 Updated.
Comment in
-
Quadrupling or quintupling inhaled corticosteroid doses to prevent asthma exacerbations: the jury's still out.Arch Dis Child Educ Pract Ed. 2019 Jun;104(3):166-167. doi: 10.1136/archdischild-2018-315608. Epub 2018 Jul 24. Arch Dis Child Educ Pract Ed. 2019. PMID: 30042131 No abstract available.
References
References to studies included in this review
Fitzgerald 2004 {published data only}
Foresi 2000 {published data only}
-
- Foresi A, Morelli MC, Catena E. Low‐dose budesonide with the addition of an increased dose during exacerbations is effective in long‐term asthma control. Chest 2000;117(2):440‐6. [PUBMED: 10669688] - PubMed
Garrett 1998 {published data only}
Harrison 2004 {published data only}
-
- Harrison TW, Oborne J, Newton S, Tattersfield AE. Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial. Lancet 2004;363(9405):271‐5. [PUBMED: 14751699] - PubMed
Martinez 2011 {published data only}
Oborne 2009 {published data only}
-
- Oborne J, Mortimer K, Hubbard RB, Tattersfield AE, Harrison TW. Quadrupling the dose of inhaled corticosteroid to prevent asthma exacerbations: a randomized, double blind, placebo controlled, parallel group, clinical trial. American Journal of Respiratory and Critical Care Medicine 2009;180(7):598‐602. [PUBMED: 19590019] - PubMed
Rice‐McDonald 2005 {published data only}
-
- Rice‐McDonald G, Bowler S, Staines G, Mitchell C. Doubling daily inhaled corticosteroid dose is ineffective in mild to moderately severe attacks of asthma in adults. Internal Medicine Journal 2005;35(12):693‐8. [PUBMED: 16313543] - PubMed
Wainwright 2009 {unpublished data only}
-
- Wainwright C. A multicentre randomised controlled trial of treatment of acute exacerbations of asthma in children with a doubling of the usual inhaled corticosteroid (ICS) dose to identify efficacy of intervention. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=630 (accessed 18 June 2015).
References to studies excluded from this review
Bateman 2008 {published data only}
-
- Bateman ED, Cheung D, Lapa e Silva J, Göhring UM, Schäfer M, Engelstätter R. Randomized comparison of ciclesonide 160 and 640 mug/day in severe asthma. Pulmonary Pharmacology and Therapeutics 2008;21(3):489‐98. [PUBMED: 18178494] - PubMed
Boushey 2005 {published data only}
-
- Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. Daily versus as‐needed corticosteroids for mild persistent asthma. New England Journal of Medicine 2005;352(15):1519‐28. [PUBMED: 15829533] - PubMed
Brand 2011 {published data only}
-
- Brand PL, Luz Garcia‐Garcia M, Morison A, Vermeulen JH, Weber HC, Brand PLP. Ciclesonide in wheezy preschool children with a positive asthma predictive index or atopy. Respiratory Medicine 2011;105(11):1588‐95. - PubMed
Bullard 1996 {published data only}
-
- Bullard MJ, Liaw SJ, Tsai YH, Min H. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. American Journal of Emergency Medicine 1996;14(2):139‐43. [PUBMED: 8924134] - PubMed
Clearie 2010 {published data only}
-
- Clearie KL. Airway challenges in different clinical phenotypes and their relationship to markers of disease and treatment [PhD thesis]. Dundee, UK: University of Dundee, 2010.
Condemi 1999 {published data only}
-
- Condemi JJ, Goldstein S, Kalberg C, Yancey S, Emmett A, Rickard K. The addition of salmeterol to fluticasone propionate versus increasing the dose of fluticasone propionate in patients with persistent asthma. Annals of Allergy, Asthma and Immunology 1999;82(4):383‐9. [PUBMED: 10227337] - PubMed
Connett 1993 {published data only}
Currie 2003 {published data only}
-
- Currie GP, Bates CE, Lee DK, Jackson CM, Lipworth BJ. Effects of fluticasone plus salmeterol versus twice the dose of fluticasone in asthmatic patients. European Journal of Clinical Pharmacology 2003;59(1):11‐5. [PUBMED: 12743669] - PubMed
De Benedictis 2005 {published data only}
-
- Benedictis FM, Giudice MM, Vetrella M, Tressanti F, Tronci A, Testi R, et al. Nebulized fluticasone propionate vs. budesonide as adjunctive treatment in children with asthma exacerbation. Journal of Asthma 2005;42(5):331‐6. [PUBMED: 16116682] - PubMed
Devidayal 1999 {published data only}
-
- Devidayal, Singhi S, Kumar L, Jayshree M. Efficacy of nebulized budesonide compared to oral prednisolone in acute bronchial asthma. Acta Paediatrica 1999;88(8):835‐40. [PUBMED: 10503681] - PubMed
Fitzgerald 2000 {published data only}
-
- FitzGerald JM, Shragge D, Haddon J, Jennings B, Math JLM, Bai T, et al. A randomized, controlled trial of high dose, inhaled budesonide versus oral prednisone in patients discharged from the emergency department following an acute asthma exacerbation. Canadian Respiratory Journal 2000;7(1):61‐7. [PUBMED: 10700672 ] - PubMed
Greening 1994 {published data only}
-
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher‐dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994;344(8917):219‐24. [PUBMED: 7913155] - PubMed
GSK 2005 {published data only}
-
- GlaxoSmithKline. A multicentre, double‐blind, randomised, four‐week, parallel‐group comparison of inhaled fluticasone propionate and inhaled beclomethasone dipropionate in patients with severe asthma controlled by high‐dose inhaled corticosteroids (FLIP04). http://www.gsk‐clinicalstudyregister.com/study/FMS30049#rs (accessed 17 December 2015).
Hedlin 1999 {published data only}
-
- Hedlin G, Svedmyr J, Ryden AC. Systemic effects of a short course of betamethasone compared with high‐dose inhaled budesonide in early childhood asthma. Acta Paediatrica 1999;88(1):48‐51. [PUBMED: 10090547 ] - PubMed
Heinig 1999 {published data only}
-
- Heinig JH, Boulet LP, Croonenborghs L, Mollers MJ. The effect of high‐dose fluticasone propionate and budesonide on lung function and asthma exacerbations in patients with severe asthma. Respiratory Medicine 1999;93(9):613‐20. [PUBMED: 10542974] - PubMed
Karpel 2007 {published data only}
-
- Karpel JP, Nayak A, Lumry W, Craig TJ, Kerwin E, Fish JE, et al. Inhaled mometasone furoate reduces oral prednisone usage and improves lung function in severe persistent asthma. Respiratory Medicine 2007;101(3):628‐37. [PUBMED: 16875813] - PubMed
La Rosa 1997 {published data only}
-
- Rosa M, Ranno C, Mandair G, Barbato A, Biraghi M. Double‐blind study of inhaled salbutamol versus salbutamol plus high‐dose flunisolide in exacerbation of bronchial asthma: a pilot study. Pediatric Asthma, Allergy and Immunology 1997;11(1):23‐30. [EMBASE: 1997208440]
Lee‐Wong 2002 {published data only}
-
- Lee‐Wong M, Dayrit FM, Kohli AR, Acquah S, Mayo PH. Comparison of high‐dose inhaled flunisolide to systemic corticosteroids in severe adult asthma. Chest 2002;122(4):1208‐13. [PUBMED: 12377843] - PubMed
Lemanske 2010 {published data only}
Leuppi 2002 {published data only}
-
- Leuppi JD, Downie SR, Salome CM, Jenkins CR, Woolcock AJ. A single high dose of inhaled corticosteroids: a possible treatment of asthma exacerbations. Swiss Medical Weekly 2002;132(1‐2):7‐11. [PUBMED: 11901445] - PubMed
Levy 1996 {published data only}
Manjra 2000 {published data only}
-
- Manjra AI, Price J, Lenney W, Hughes S, Barnacle H. Efficacy of nebulized fluticasone propionate compared with oral prednisolone in children with an acute exacerbation of asthma. Respiratory Medicine 2000;94(12):1206‐14. [PUBMED: 11192957] - PubMed
Matz 2001 {published data only}
-
- Matz J, Emmett A, Rickard K, Kalberg C. Addition of salmeterol to low‐dose fluticasone versus higher‐dose fluticasone: an analysis of asthma exacerbations. Journal of Allergy and Clinical Immunology 2001;107(5):783‐9. [PUBMED: 11344343] - PubMed
Milani 2004 {published data only}
-
- Milani GK, Rosario Filho NA, Riedi CA, Figueiredo BC. Nebulized budesonide to treat acute asthma in children. Jornal de Pediatria 2004;80(2):106‐12. [EMBASE: 2006186583] - PubMed
Nana 1998 {published data only}
-
- Nana A, Youngchaiyud P, Charoenratanakul S, Boe J, Lofdahl C, Selroos O, et al. High‐dose inhaled budesonide may substitute for oral therapy after an acute asthma attack. Journal of Asthma 1998;35(8):647‐55. [PUBMED: 9860085] - PubMed
Nuhoglu 2001 {published data only}
-
- Nuhoglu Y, Bahceciler NN, Barlan IB, Mujdat Basaran M. The effectiveness of high‐dose inhaled budesonide therapy in the treatment of acute asthma exacerbations in children. Annals of Allergy, Asthma, and Immunology 2001;86(3):318‐22. [PUBMED: 11289332] - PubMed
O'Connor 2010 {published data only}
-
- O'Connor BJ, Kilfeather S, Cheung D, Kafé H, Blagden MD, Schlösser N, et al. Efficacy and safety of ciclesonide in patients with severe asthma: a 12‐week, double‐blind, randomized, parallel‐group study with long‐term (1‐year) follow‐up. Expert Opinion on Pharmacotherapy 2010;11(17):2791‐803. [DOI: 10.1517/14656566.2010.526603] - DOI - PubMed
Pedersen 2009 {published data only}
-
- Pedersen S, Engelstätter R, Weber HJ, Hirsch S, Barkai L, Emeryk A, et al. Efficacy and safety of ciclesonide once daily and fluticasone propionate twice daily in children with asthma. Pulmonary Pharmacology and Therapeutics 2009;22(3):214‐20. - PubMed
Razi 2008 {published data only}
-
- Razi CH, Turktas I, Bakirtas A. Comparison of single 2000‐microg dose treatment vs. sequential repeated‐dose 500‐microg treatments with nebulized budesonide in acute asthma exacerbations. Annals of Allergy, Asthma, and Immunology 2008;100(4):370‐6. [PUBMED: 18450124] - PubMed
Rodrigo 1998 {published data only}
-
- Rodrigo G, Rodrigo C. Inhaled flunisolide for acute severe asthma. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Pt 1):698‐703. [PUBMED: 9517578] - PubMed
Rodrigo 2005 {published data only}
-
- Rodrigo GJ. Comparison of inhaled fluticasone with intravenous hydrocortisone in the treatment of adult acute asthma. American Journal of Respiratory and Critical Care Medicine 2005;171(11):1231‐6. [PUBMED: 15764724] - PubMed
Schuh 2000 {published data only}
-
- Schuh S, Reisman J, Alshehri M, Dupuis A, Corey M, Arseneault R, et al. A comparison of inhaled fluticasone and oral prednisone for children with severe acute asthma. New England Journal of Medicine 2000;343(10):689‐94. [PUBMED: 10974132] - PubMed
Schuh 2006 {published data only}
-
- Schuh S, Dick PT, Stephens D, Hartley M, Khaikin S, Rodrigues L, et al. High‐dose inhaled fluticasone does not replace oral prednisolone in children with mild to moderate acute asthma. Pediatrics 2006;118(2):644‐50. [PUBMED: 16882819] - PubMed
Sekerel 2005 {published data only}
-
- Sekerel BE, Sackesen C, Tuncer A, Adalioglu G. The effect of nebulized budesonide treatment in children with mild to moderate exacerbations of asthma. Acta Paediatrica 2005;94(10):1372‐7. [PUBMED: 16299866] - PubMed
Singhi 1999 {published data only}
-
- Singhi S, Banerjee S, Nanjundaswamy H. Inhaled budesonide in acute asthma. Journal of Paediatrics and Child Health 1999;35(5):483‐7. [PUBMED: 10571764] - PubMed
Svedmyr 1995 {published data only}
-
- Svedmyr J, Nyberg E, Asbrink Nilsson E, Hedlin G. Intermittent treatment with inhaled steroids for deterioration of asthma due to upper respiratory tract infections. Acta Paediatrica 1995;84(8):884‐8. [PUBMED: 7488811] - PubMed
Volovitz 1998 {published data only}
-
- Volovitz B, Bentur L, Finkelstein Y, Mansour Y, Shalitin S, Nussinovitch M, et al. Effectiveness and safety of inhaled corticosteroids in controlling acute asthma attacks in children who were treated in the emergency department: a controlled comparative study with oral prednisolone. Journal of Allergy and Clinical Immunology 1998;102(4 Pt 1):605‐9. [PUBMED: 9802368 ] - PubMed
Wilson 1990 {published data only}
Yousef 2012 {published data only}
-
- Yousef E, Hossain J, Mannan S, Skorpinski E, McGeady S. Early intervention with high‐dose inhaled corticosteroids for control of acute asthma exacerbations at home and improved outcomes: a randomized controlled trial. Allergy and Asthma Proceedings 2012;33(6):508‐13. - PubMed
-
- Yousef E, Hossain J, Mannnan S. Ineffectiveness of high‐dose inhaled corticosteroids for control of pre‐exacerbation asthma symptoms. Journal of Allergy and Clinical Immunology (Abstract) 2011;127(2 Suppl 1):AB85.
References to ongoing studies
NCT02066129 {published data only}
-
- Step‐up Yellow Zone Inhaled Corticosteroids to Prevent Exacerbations. https://clinicaltrials.gov/ct2/show/NCT02066129 (accessed 10 June 2015).
Additional references
Adams 1999
Barnes 1993
-
- Barnes NC, Marone G, Maria GU, Visser S, Utama I, Payne SL. A comparison of fluticasone propionate, 1 mg daily, with beclomethasone dipropionate, 2 mg daily, in the treatment of severe asthma. European Respiratory Journal 1993;6:877‐85. [PUBMED: 8339809] - PubMed
Belda 2007
-
- Belda J, Margarit G, Martínez C, Bellido‐Casado J, Casan P, Torrejón M, et al. Anti‐inflammatory effects of high‐dose inhaled fluticasone versus oral prednisone in asthma exacerbations. European Respiratory Journal 2007;30(6):1143‐9. [PUBMED: 17690122] - PubMed
Boulet 1999
BTS 1997
-
- British Thoracic Society, The National Asthma Campaign, The Royal College of Physicians of London in association with The General Practitioner in Asthma Group, The British Association of Accident and Emergency Medicine, The British Paediatric Respiratory Society, and the Royal College of Paediatrics and Child Health. British guidelines on asthma management. Thorax 1997;52(Suppl 1):S1‐21. - PubMed
BTS/SIGN 2014
-
- British Thoracic Society/Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. http://sign.ac.uk/pdf/SIGN141.pdf (accessed 17 Feb 2015).
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Ducharme 2009
-
- Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Preemptive use of high‐dose fluticasone for virus‐induced wheezing in young children. New England Journal of Medicine 2009;360(4):339‐53. - PubMed
Edmonds 2003
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Fanta 1983
-
- Fanta CH, Rossing TH, McFadden ER Jr. Glucocorticoids in acute asthma. A critical controlled trial. American Journal of Medicine 1983;74(5):845‐51. [PUBMED: 6340496] - PubMed
Fields 2001
-
- Fields AP. Meta‐analysis of correlation coefficients: a Monte Carlo comparison of fixed‐ and random‐effects methods. Psychological Methods 2001;6(2):161‐80. - PubMed
GINA 2015
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention. http://www.ginasthma.org/local/uploads/files/GINA_Report_2015.pdf (accessed 17 Feb 2015).
Global Asthma Report 2014
-
- Global Asthma Network. Global Asthma Report 2015. http://www.globalasthmareport.org/resources/Global_Asthma_Report_2014.pdf (accessed 10 March 2016).
Greenland 1985
-
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41:55‐68. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnston 2006
Lane 2006
-
- Lane S, Molina J, Plusa T. An international observational prospective study to determine the cost of asthma exacerbations (COAX). Respiratory Medicine 2006;100(3):434‐50. [PUBMED: 16099149] - PubMed
Lemiere 2003
-
- Lemiere C, Bai T, Balter M, Bayliff C, Becker A, Boulet LP, et al. Adult Asthma Consensus Guidelines Update 2003. Canadian Respiratory Journal May/June 2004;11(Suppl A):9A‐33A. - PubMed
Lloyd 2007
McEvoy 2000
-
- McEvoy CE, Niewoehner DE. Corticosteroids in chronic obstructive pulmonary disease. Clinical benefits and risks. Clinical Chest Medicine 2000;21(4):739‐52. [PUBMED: 11194783] - PubMed
NHIS 2005
-
- National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention 2005. National Health Interview Survey (NHIS 2005). Hyattsville, MD. http://www.cdc.gov/nchs/about/major/nhis/reports_2005.htm (accessed 14 March 2008).
NHLBI 2007
-
- National Heart, Lung, and Blood Institute. Guidelines for the diagnosis and management of asthma (EPR‐3). Bethesda, MD: National Institutes of Health, 2007. Publication no. 08‐4051.
Oommen 2003
-
- Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent‐initiated oral prednisolone for viral wheeze in children aged 1‐5 years; randomised controlled trial. Lancet 2003;362(9394):1433‐8. [PUBMED: 14602435 ] - PubMed
Panickar 2009
-
- Panickar J, Lakhanpaul M, Lambert PC, Kenia P, Stephenson T, Smyth A, et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. New England Journal of Medicine 2009;360:329‐38. - PubMed
Pollack 2002
-
- Pollack CV Jr, Pollack ES, Baren JM, Smith SR, Woodruff PG, Clark S, et al. Multicenter Airway Research Collaboration Investigators. A prospective multicenter study of patient factors associated with hospital admission from the emergency department among children with acute asthma. Archives of Pediatric & Adolescent Medicine 2002;156(9):934‐40. [PUBMED: 12197803] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodrigo 2006
-
- Rodrigo GJ. Rapid effects of inhaled corticosteroids in acute asthma: an evidence‐based evaluation. Chest 2006;130(5):1301‐11. [PUBMED: 17099004] - PubMed
Rowe 2001
Sears 2000
-
- Sears M. Natural history and epidemiology. In: Fitzgerald JM, Ernst P, Boulet L‐P, O'Byrne PM editor(s). Evidence based asthma management. Hamilton: BC Decker Inc., 2000:1‐12.
Stovold 2014
Turner 1998
-
- Turner MO, Taylor D, Bennett R, Fitzgerald JM. A randomized trial comparing peak expiratory flow and symptom self‐management plans for patients with asthma attending a primary care clinic. American Journal of Respiratory and Critical Care Medicine 1998;157:540‐6. - PubMed
Wang 2007
-
- Wang R, Lagakos SW, Hare JH, Hunter DJ, Drazen JM. Special report. Statistics in medicine ‐ reporting of subgroup analyses in clinical trials. New England Journal of Medicine 2007;357(21):2189‐94. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

