Human glia can both induce and rescue aspects of disease phenotype in Huntington disease
- PMID: 27273432
- PMCID: PMC4899632
- DOI: 10.1038/ncomms11758
Human glia can both induce and rescue aspects of disease phenotype in Huntington disease
Abstract
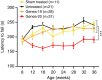
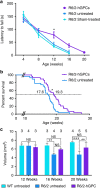
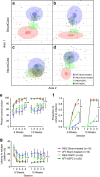
The causal contribution of glial pathology to Huntington disease (HD) has not been heavily explored. To define the contribution of glia to HD, we established human HD glial chimeras by neonatally engrafting immunodeficient mice with mutant huntingtin (mHTT)-expressing human glial progenitor cells (hGPCs), derived from either human embryonic stem cells or mHTT-transduced fetal hGPCs. Here we show that mHTT glia can impart disease phenotype to normal mice, since mice engrafted intrastriatally with mHTT hGPCs exhibit worse motor performance than controls, and striatal neurons in mHTT glial chimeras are hyperexcitable. Conversely, normal glia can ameliorate disease phenotype in transgenic HD mice, as striatal transplantation of normal glia rescues aspects of electrophysiological and behavioural phenotype, restores interstitial potassium homeostasis, slows disease progression and extends survival in R6/2 HD mice. These observations suggest a causal role for glia in HD, and further suggest a cell-based strategy for disease amelioration in this disorder.
Conflict of interest statement
Drs Goldman and Windrem hold a patent on human glial chimeric mice, US 7,524,491, from which they receive no financial remuneration. None of the authors have relevant financial sakes in this work. Drs Curtin and Brunner are employees of Psychogenics, Inc.
Figures



Comment in
-
Neurodegenerative disease: Glial cells - friend and foe in Huntington disease?Nat Rev Neurol. 2016 Aug;12(8):430-1. doi: 10.1038/nrneurol.2016.95. Epub 2016 Jun 24. Nat Rev Neurol. 2016. PMID: 27340025 No abstract available.
References
-
- Di Giorgio F. P., Boulting G. L., Bobrowicz S. & Eggan K. C. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell 3, 637–648 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

