Characterizing treatment pathways at scale using the OHDSI network
- PMID: 27274072
- PMCID: PMC4941483
- DOI: 10.1073/pnas.1510502113
Characterizing treatment pathways at scale using the OHDSI network
Abstract
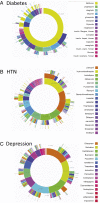
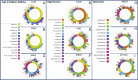
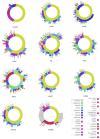
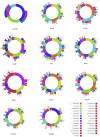
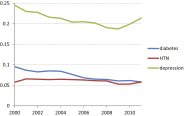
Observational research promises to complement experimental research by providing large, diverse populations that would be infeasible for an experiment. Observational research can test its own clinical hypotheses, and observational studies also can contribute to the design of experiments and inform the generalizability of experimental research. Understanding the diversity of populations and the variance in care is one component. In this study, the Observational Health Data Sciences and Informatics (OHDSI) collaboration created an international data network with 11 data sources from four countries, including electronic health records and administrative claims data on 250 million patients. All data were mapped to common data standards, patient privacy was maintained by using a distributed model, and results were aggregated centrally. Treatment pathways were elucidated for type 2 diabetes mellitus, hypertension, and depression. The pathways revealed that the world is moving toward more consistent therapy over time across diseases and across locations, but significant heterogeneity remains among sources, pointing to challenges in generalizing clinical trial results. Diabetes favored a single first-line medication, metformin, to a much greater extent than hypertension or depression. About 10% of diabetes and depression patients and almost 25% of hypertension patients followed a treatment pathway that was unique within the cohort. Aside from factors such as sample size and underlying population (academic medical center versus general population), electronic health records data and administrative claims data revealed similar results. Large-scale international observational research is feasible.
Keywords: data network; observational research; treatment pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Etheredge LM. Rapid learning: A breakthrough agenda. Health Aff (Millwood) 2014;33(7):1155–1162. - PubMed
-
- Guyatt G, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: Report from an american college of chest physicians task force. Chest. 2006;129(1):174–181. - PubMed
-
- Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878–1886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

