A randomized, parallel-group study to evaluate the efficacy of umeclidinium/vilanterol 62.5/25 μg on health-related quality of life in patients with COPD
- PMID: 27274218
- PMCID: PMC4869636
- DOI: 10.2147/COPD.S102962
A randomized, parallel-group study to evaluate the efficacy of umeclidinium/vilanterol 62.5/25 μg on health-related quality of life in patients with COPD
Abstract
Background: The combination of the inhaled muscarinic antagonist umeclidinium (UMEC) with the long-acting β2-agonist vilanterol (VI) has been shown to provide significant improvements in lung function compared with UMEC, VI, or placebo (PBO) in patients with chronic obstructive pulmonary disease (COPD). This study was specifically designed to support these findings by assessing health-related quality of life and symptomatic outcomes in a similar population.
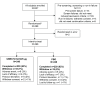
Methods: This was a 12-week multicenter, randomized, double-blind, parallel-group, placebo-controlled study. Eligible patients were randomized 1:1 to receive once-daily UMEC/VI 62.5/25 μg (via ELLIPTA(®) dry powder inhaler) or PBO for 12 weeks. The primary endpoint was St George's Respiratory Questionnaire (SGRQ) total score at day 84. Secondary efficacy endpoints included rescue albuterol use (puffs/day) over weeks 1-12 and trough forced expiratory volume in 1 second on day 84. Adverse events were also assessed.
Results: A total of 496 patients were included in the intent-to-treat population in the UMEC/VI (n=248) and PBO (n=248) treatment groups. UMEC/VI 62.5/25 μg provided a significant and clinically meaningful improvement in SGRQ total score at day 84 versus PBO (difference between treatments in SGRQ total score change from baseline: -4.03 [95% confidence interval {CI}: -6.28, -1.79]; P<0.001). UMEC/VI 62.5/25 μg resulted in a statistically significant reduction in rescue albuterol use versus PBO (-0.7 puffs/day [95% CI: -1.1, -0.4]; P<0.001). UMEC/VI 62.5/25 μg provided a significant and clinically meaningful improvement in trough forced expiratory volume in 1 second on day 84 versus PBO (122 mL [95% CI: 71, 172]; P<0.001). The incidence of adverse events was similar between treatments (32% and 30% of patients in the UMEC/VI 62.5/25 μg and PBO groups, respectively).
Conclusion: The results of this study demonstrate that treatment with UMEC/VI 62.5/25 μg provides clinically important improvements in SGRQ and rescue medication use versus PBO in patients with moderate-to-very-severe COPD.
Keywords: COPD; SGRQ; health-related quality of life; long-acting bronchodilator; umeclidinium; vilanterol.
Figures



References
-
- Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107(10):1538–1546. - PubMed
-
- GOLD Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2015. [Accessed March 10, 2015]. Available from: http://www.goldcopd.com/
-
- Gudmundsson G, Gislason T, Janson C, et al. Depression, anxiety and health status after hospitalisation for COPD: a multicentre study in the Nordic countries. Respir Med. 2006;100(1):87–93. - PubMed
-
- Hanania NA, Mullerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183(5):604–611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

