The Chemistry of Gut Microbial Metabolism of Polyphenols
- PMID: 27274718
- PMCID: PMC4888912
- DOI: 10.1007/s11101-016-9459-z
The Chemistry of Gut Microbial Metabolism of Polyphenols
Abstract
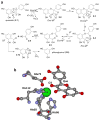
Gut microbiota contribute to the metabolism of dietary polyphenols and affect the bioavailability of both the parent polyphenols and their metabolites. Although there is a large number of reports of specific polyphenol metabolites, relatively little is known regarding the chemistry and enzymology of the metabolic pathways utilized by specific microbial species and taxa, which is the focus of this review. Major classes of dietary polyphenols include monomeric and oligomeric catechins (proanthocyanidins), flavonols, flavanones, ellagitannins, and isoflavones. Gut microbial metabolism of representatives of these polyphenol classes can be classified as A- and C-ring cleavage (retro Claisen reactions), C-ring cleavage mediated by dioxygenases, dehydroxylations (decarboxylation or reduction reactions followed by release of H2O molecules), and hydrogenations of alkene moieties in polyphenols, such as resveratrol, curcumin, and isoflavones (mediated by NADPH-dependent reductases). The qualitative and quantitative metabolic output of the gut microbiota depends to a large extent on the metabolic capacity of individual taxa, which emphasizes the need for assessment of functional analysis in conjunction with determinations of gut microbiota compositions.
Keywords: Catabolism; flavonoid; gut microbiota; mechanism; metabolic pathway.
Figures
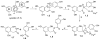


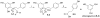
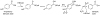

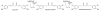

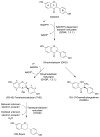


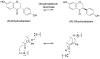

References
-
- Appeldoorn MM, Vincken JP, Aura AM, et al. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-gamma-valerolactone as the major metabolites. J Agric Food Chem. 2009;57:1084–1092. - PubMed
-
- Aura AM, Martin-Lopez P, O’Leary KA, et al. In vitro metabolism of anthocyanins by human gut microflora. Eur J Nutr. 2005;44:133–142. - PubMed
-
- Barroso E, Sanchez-Patan F, Martin-Alvarez PJ, et al. Lactobacillus plantarum IFPL935 favors the initial metabolism of red wine polyphenols when added to a colonic microbiota. J Agric Food Chem. 2013;61:10163–10172. - PubMed
-
- Bitsch I, Janssen M, Netzel M, et al. Bioavailability of anthocyanidin-3-glycosides following consumption of elderberry extract and blackcurrant juice. Int J Clin Pharmacol Ther. 2004;42:293–300. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
