Biomaterial Applications in Cell-Based Therapy in Experimental Stroke
- PMID: 27274738
- PMCID: PMC4870368
- DOI: 10.1155/2016/6810562
Biomaterial Applications in Cell-Based Therapy in Experimental Stroke
Abstract
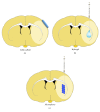
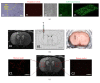
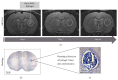
Stroke is an important health issue corresponding to the second cause of mortality and first cause of severe disability with no effective treatments after the first hours of onset. Regenerative approaches such as cell therapy provide an increase in endogenous brain structural plasticity but they are not enough to promote a complete recovery. Tissue engineering has recently aroused a major interesting development of biomaterials for use into the central nervous system. Many biomaterials have been engineered based on natural compounds, synthetic compounds, or a mix of both with the aim of providing polymers with specific properties. The mechanical properties of biomaterials can be exquisitely regulated forming polymers with different stiffness, modifiable physical state that polymerizes in situ, or small particles encapsulating cells or growth factors. The choice of biomaterial compounds should be adapted for the different applications, structure target, and delay of administration. Biocompatibilities with embedded cells and with the host tissue and biodegradation rate must be considerate. In this paper, we review the different applications of biomaterials combined with cell therapy in ischemic stroke and we explore specific features such as choice of biomaterial compounds and physical and mechanical properties concerning the recent studies in experimental stroke.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

