Randomized Phase II Study of Docetaxel plus Personalized Peptide Vaccination versus Docetaxel plus Placebo for Patients with Previously Treated Advanced Wild Type EGFR Non-Small-Cell Lung Cancer
- PMID: 27274999
- PMCID: PMC4870343
- DOI: 10.1155/2016/1745108
Randomized Phase II Study of Docetaxel plus Personalized Peptide Vaccination versus Docetaxel plus Placebo for Patients with Previously Treated Advanced Wild Type EGFR Non-Small-Cell Lung Cancer
Abstract
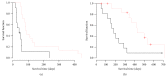
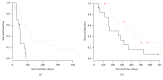
Objectives. To evaluate the efficacy and safety of personalized peptide vaccination (PPV) combined with chemotherapy for patients with previously treated advanced non-small-cell lung cancer (NSCLC). Patients and Methods. Previously treated PS0-1 patients with IIIB/IV EGFR (epidermal growth factor receptor) wild genotype NSCLC were randomly assigned to docetaxel (60 mg/m(2) on Day 1) plus PPV based on preexisting host immunity or docetaxel plus placebo. Docetaxel administration was repeated every 3 weeks until disease progression. Personalized peptides or placebo was injected subcutaneously weekly in the first 8 weeks and biweekly in subsequent 16 weeks. The primary efficacy endpoint was progression-free survival (PFS). Results. PPV related toxicity was grade 2 or less skin reaction. The median PFS for placebo arm and PPV arm was 52 days and 59 days, respectively. There was no significant difference between two arms by log-rank test (p = 0.42). Interestingly, PFS and overall survival (OS) in humoral immunological responder were significantly longer than those in nonresponder. Conclusion. PPV did not improve the survival in combination with docetaxel for previously treated advanced NSCLC. However, PPV may be efficacious for the humoral immunological responders and a further clinical investigation is needed.
Figures

References
-
- Fossella F. V., DeVore R., Kerr R. N., et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. Journal of Clinical Oncology. 2000;18(12):2354–2362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

