Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101
- PMID: 27275632
- PMCID: PMC5210022
- DOI: 10.1001/jamasurg.2016.1137
Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101
Abstract
Importance: Although consensus statements support the preoperative treatment of borderline resectable pancreatic cancer, no prospective, quality-controlled, multicenter studies of this strategy have been conducted. Existing studies are retrospective and confounded by heterogeneity in patients studied, therapeutic algorithms used, and outcomes reported.
Objective: To determine the feasibility of conducting studies of multimodality therapy for borderline resectable pancreatic cancer in the cooperative group setting.
Design, setting, and participants: A prospective, multicenter, single-arm trial of a multimodality treatment regimen administered within a study framework using centralized quality control with the cooperation of 14 member institutions of the National Clinical Trials Network. Twenty-nine patients with biopsy-confirmed pancreatic cancer preregistered, and 23 patients with tumors who met centrally reviewed radiographic criteria registered. Twenty-two patients initiated therapy (median age, 64 years [range, 50-76 years]; 55% female). Patients registered between May 29, 2013, and February 7, 2014.
Interventions: Patients received modified FOLFIRINOX treatment (85 mg/m2 of oxaliplatin, 180 mg/m2 of irinotecan hydrochloride, 400 mg/m2 of leucovorin calcium, and then 2400 mg/m2 of 5-fluorouracil for 4 cycles) followed by 5.5 weeks of external-beam radiation (50.4 Gy delivered in 28 daily fractions) with capecitabine (825 mg/m2 orally twice daily) prior to pancreatectomy.
Main outcomes and measures: Feasibility, defined by the accrual rate, the safety of the preoperative regimen, and the pancreatectomy rate.
Results: The accrual rate of 2.6 patients per month was superior to the anticipated rate. Although 14 of the 22 patients (64% [95% CI, 41%-83%]) had grade 3 or higher adverse events, 15 of the 22 patients (68% [95% CI, 49%-88%]) underwent pancreatectomy. Of these 15 patients, 12 (80%) required vascular resection, 14 (93%) had microscopically negative margins, 5 (33%) had specimens that had less than 5% residual cancer cells, and 2 (13%) had specimens that had pathologic complete responses. The median overall survival of all patients was 21.7 months (95% CI, 15.7 to not reached) from registration.
Conclusions and relevance: The successful completion of this collaborative study demonstrates the feasibility of conducting quality-controlled trials for this disease stage in the multi-institutional setting. The data generated by this study and the logistical elements that facilitated the trial's completion are currently being used to develop cooperative group trials with the goal of improving outcomes for this subset of patients.
Trial registration: clinicaltrials.gov Identifier: NCT01821612.
Conflict of interest statement
Disclosures: None reported.
Figures
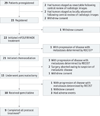
An additional patient had progression of disease determined by RECIST owing to isolated progression after mFOLFIRINOX treatment but, per protocol, proceeded to chemoradiation.
Treatment was halted for 1 patient after first cycle of postoperative chemotherapy.

Comment in
-
Borderline Resectable Pancreatic Cancer: Answering the Most Important Question First.JAMA Surg. 2016 Aug 17;151(8):e161150. doi: 10.1001/jamasurg.2016.1150. Epub 2016 Aug 17. JAMA Surg. 2016. PMID: 27276510 No abstract available.
References
-
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13(8):1035–1046. - PubMed
-
- Yamada S, Fujii T, Sugimoto H, et al. Aggressive surgery for borderline resectable pancreatic cancer: evaluation of National Comprehensive Cancer Network guidelines. Pancreas. 2013;42(6):1004–1010. - PubMed
-
- Nakao A, Kanzaki A, Fujii T, et al. Correlation between radiographic classification and pathological grade of portal vein wall invasion in pancreatic head cancer. Ann Surg. 2012;255(1):103–108. - PubMed
-
- Abrams RA, Lowy AM, O’Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1751–1756. - PubMed
-
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma, Version 2.2015. Fort Washington, PA: NCCN; 2015.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA233270/CA/NCI NIH HHS/United States
- U10 CA180802/CA/NCI NIH HHS/United States
- U10 CA180835/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180858/CA/NCI NIH HHS/United States
- U10 CA180836/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
- P30 CA022453/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- UG1 CA233196/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- U10 CA037429/CA/NCI NIH HHS/United States
- UG1 CA189870/CA/NCI NIH HHS/United States
- U10 CA180847/CA/NCI NIH HHS/United States
- U10 CA180850/CA/NCI NIH HHS/United States
- U10 CA180799/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

