18O-Tracer Metabolomics Reveals Protein Turnover and CDP-Choline Cycle Activity in Differentiating 3T3-L1 Pre-Adipocytes
- PMID: 27275782
- PMCID: PMC4898700
- DOI: 10.1371/journal.pone.0157118
18O-Tracer Metabolomics Reveals Protein Turnover and CDP-Choline Cycle Activity in Differentiating 3T3-L1 Pre-Adipocytes
Abstract
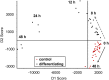
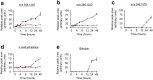
The differentiation of precursor cells into mature adipocytes (adipogenesis) has been an area of increased focus, spurred by a rise in obesity rates. Though our understanding of adipogenesis and its regulation at the cellular level is growing, many questions remain, especially regarding the regulation of the metabolome. The 3T3-L1 cell line is the most well characterized cellular model of adipogenesis. Using a time course metabolomics approach, we show that the 3T3-L1 preadipocyte metabolome is greatly altered during the first 48 hours of differentiation, where cells go through about two rounds of cell division, a process known as mitotic clonal expansion. Short-chain peptides were among several small molecules that were increased during mitotic clonal expansion. Additional indicators of protein turnover were also increased, including bilirubin, a degradation product of heme-containing proteins, and 3-methylhistidine, a post-translationally modified amino acid that is not reutilized for protein synthesis. To study the origin of the peptides, we treated differentiating preadipocytes with 18O labeled water and found that 18O was incorporated into the short chain peptides, confirming them, at least in part, as products of hydrolysis. Inhibitors of the proteasome or matrix metalloproteinases affected the peptide levels during differentiation, but inhibitors of autophagy or peptidases did not. 18O was also incorporated into several choline metabolites including cytidine 5'-diphosphocholine (CDP-choline), glycerophosphocholine, and several phosphatidylcholine species, indicative of phosphatidylcholine synthesis/degradation and of flux through the CDP-choline cycle, a hallmark of proliferating cells. 18O-Tracer metabolomics further showed metabolic labeling of glutamate, suggestive of glutaminolysis, also characteristic of proliferating cells. Together, these results highlight the utility of 18O isotope labeling in combination with metabolomics to uncover changes in cellular metabolism that are not detectable by time-resolved metabolomics.
Conflict of interest statement
Figures







References
-
- Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev. 1995;5(5):571–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

