A Computational Model of YAP/TAZ Mechanosensing
- PMID: 27276271
- PMCID: PMC4922562
- DOI: 10.1016/j.bpj.2016.04.040
A Computational Model of YAP/TAZ Mechanosensing
Abstract
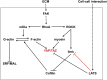
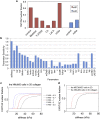
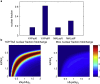
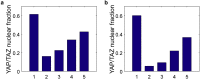
In cell proliferation, stem cell differentiation, chemoresistance, and tissue organization, the ubiquitous role of YAP/TAZ continues to impact our fundamental understanding in numerous physiological and disease systems. YAP/TAZ is an important signaling nexus integrating diverse mechanical and biochemical signals, such as ECM stiffness, adhesion ligand density, or cell-cell contacts, and thus strongly influences cell fate. Recent studies show that YAP/TAZ mechanical sensing is dependent on RhoA-regulated stress fibers. However, current understanding of YAP/TAZ remains limited due to the unknown interaction between the canonical Hippo pathway and cell tension. Furthermore, the multiscale relationship connecting adhesion signaling to YAP/TAZ activity through cytoskeleton dynamics remains poorly understood. To identify the roles of key signaling molecules in mechanical signal sensing and transduction, we present a, to our knowledge, novel computational model of the YAP/TAZ signaling pathway. This model converts extracellular-matrix mechanical properties to biochemical signals via adhesion, and integrates intracellular signaling cascades associated with cytoskeleton dynamics. We perform perturbations of molecular levels and sensitivity analyses to predict how various signaling molecules affect YAP/TAZ activity. Adhesion molecules, such as FAK, are predicted to rescue YAP/TAZ activity in soft environments via the RhoA pathway. We also found that changes of molecule concentrations result in different patterns of YAP/TAZ stiffness response. We also investigate the sensitivity of YAP/TAZ activity to ECM stiffness, and compare with that of SRF/MAL, which is another important regulator of differentiation. In addition, the model shows that the unresolved synergistic effect of YAP/TAZ activity between the mechanosensing and the Hippo pathways can be explained by the interaction of LIM-kinase and LATS. Overall, our model provides a, to our knowledge, novel platform for studying YAP/TAZ activity in the context of integrating different signaling pathways. This platform can be used to gain, to our knowledge, new fundamental insights into roles of key molecular and mechanical regulators on development, tissue engineering, or tumor progression.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Piccolo S., Dupont S., Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 2014;94:1287–1312. - PubMed
-
- Low B.C., Pan C.Q., Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588:2663–2670. - PubMed
-
- Goulev Y., Fauny J.D., Zider A. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr. Biol. 2008;18:435–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

