Characterizing spatiotemporal information loss in sparse-sampling-based dynamic MRI for monitoring respiration-induced tumor motion in radiotherapy
- PMID: 27277029
- PMCID: PMC4866962
- DOI: 10.1118/1.4948684
Characterizing spatiotemporal information loss in sparse-sampling-based dynamic MRI for monitoring respiration-induced tumor motion in radiotherapy
Abstract
Purpose: Sparse-sampling and reconstruction techniques represent an attractive strategy to achieve faster image acquisition speeds, while maintaining adequate spatial resolution and signal-to-noise ratio in rapid magnetic resonance imaging (MRI). The authors investigate the use of one such sequence, broad-use linear acquisition speed-up technique (k-t BLAST) in monitoring tumor motion for thoracic and abdominal radiotherapy and examine the potential trade-off between increased sparsification (to increase imaging speed) and the potential loss of "true" information due to greater reliance on a priori information.
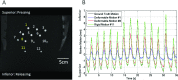
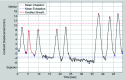
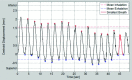
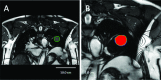
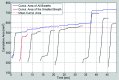
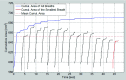
Methods: Lung tumor motion trajectories in the superior-inferior direction, previously recorded from ten lung cancer patients, were replayed using a motion phantom module driven by an MRI-compatible motion platform. Eppendorf test tubes filled with water which serve as fiducial markers were placed in the phantom. The modeled rigid and deformable motions were collected in a coronal image slice using balanced fast field echo in conjunction with k-t BLAST. Root mean square (RMS) error was used as a metric of spatial accuracy as measured trajectories were compared to input data. The loss of spatial information was characterized for progressively increasing acceleration factor from 1 to 16; the resultant sampling frequency was increased approximately from 2.5 to 19 Hz when the principal direction of the motion was set along frequency encoding direction. In addition to the phantom study, respiration-induced tumor motions were captured from two patients (kidney tumor and lung tumor) at 13 Hz over 49 s to demonstrate the impact of high speed motion monitoring over multiple breathing cycles. For each subject, the authors compared the tumor centroid trajectory as well as the deformable motion during free breathing.
Results: In the rigid and deformable phantom studies, the RMS error of target tracking at the acquisition speed of 19 Hz was approximately 0.3-0.4 mm, which was smaller than the reconstructed pixel resolution of 0.67 mm. In the patient study, the dynamic 2D MRI enabled the monitoring of cycle-to-cycle respiratory variability present in the tumor position. It was seen that the range of centroid motion as well as the area covered due to target motion during each individual respiratory cycle was underestimated compared to the entire motion range observed over multiple breathing cycles.
Conclusions: The authors' initial results demonstrate that sparse-sampling- and reconstruction-based dynamic MRI can be used to achieve adequate image acquisition speeds without significant information loss for the task of radiotherapy guidance. Such monitoring can yield spatial and temporal information superior to conventional offline and online motion capture methods used in thoracic and abdominal radiotherapy.
Figures



Similar articles
-
Quantifying the impact of respiratory-gated 4D CT acquisition on thoracic image quality: a digital phantom study.Med Phys. 2015 Jan;42(1):324-34. doi: 10.1118/1.4903936. Med Phys. 2015. PMID: 25563272
-
Geometric validation of self-gating k-space-sorted 4D-MRI vs 4D-CT using a respiratory motion phantom.Med Phys. 2015 Oct;42(10):5787-97. doi: 10.1118/1.4929552. Med Phys. 2015. PMID: 26429253 Free PMC article.
-
Quantifying the accuracy of the tumor motion and area as a function of acceleration factor for the simulation of the dynamic keyhole magnetic resonance imaging method.Med Phys. 2016 May;43(5):2639. doi: 10.1118/1.4947508. Med Phys. 2016. PMID: 27147373
-
[Image-guided radiotherapy in lung cancer].Cancer Radiother. 2018 Oct;22(6-7):602-607. doi: 10.1016/j.canrad.2018.06.009. Epub 2018 Aug 10. Cancer Radiother. 2018. PMID: 30104150 Review. French.
-
[Investigation of respiratory-dependent movements of pulmonary space-occupying lesions with MRI].Radiologe. 2009 Aug;49(8):705-11. doi: 10.1007/s00117-009-1882-3. Radiologe. 2009. PMID: 19693619 Review. German.
Cited by
-
Intensity-modulated proton therapy (IMPT) interplay effect evaluation of asymmetric breathing with simultaneous uncertainty considerations in patients with non-small cell lung cancer.Med Phys. 2020 Nov;47(11):5428-5440. doi: 10.1002/mp.14491. Epub 2020 Oct 13. Med Phys. 2020. PMID: 32964474 Free PMC article.
-
Clinical evaluation of 4D MRI in the delineation of gross and internal tumor volumes in comparison with 4DCT.J Appl Clin Med Phys. 2019 Sep;20(9):51-60. doi: 10.1002/acm2.12699. J Appl Clin Med Phys. 2019. PMID: 31538719 Free PMC article.
References
-
- Mageras G. S., Pevsner A., Yorke E. D., Rosenzweig K. E., Ford E. C., Hertanto A., Larson S. M., Lovelock D. M., Erdi Y. E., Nehmeh S. A., Humm J. L., and Ling C. C., “Measurement of lung tumor motion using respiration-correlated CT,” Int. J. Radiat. Oncol., Biol., Phys. 60, 933–941 (2004).10.1016/S0360-3016(04)01078-8 - DOI - PubMed
-
- Keall P. J., Mageras G. S., Balter J. M., Emery R. S., Forster K. M., Jiang S. B., Kapatoes J. M., Low D. A., Murphy M. J., Murray B. R., Ramsey C. R., Van Herk M. B., Vedam S. S., Wong J. W., and Yorke E., “The management of respiratory motion in radiation oncology report of AAPM Task Group 76,” Med. Phys. 33, 3874–3900 (2006).10.1118/1.2349696 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

