Semiautomated segmentation of head and neck cancers in 18F-FDG PET scans: A just-enough-interaction approach
- PMID: 27277044
- PMCID: PMC4874930
- DOI: 10.1118/1.4948679
Semiautomated segmentation of head and neck cancers in 18F-FDG PET scans: A just-enough-interaction approach
Abstract
Purpose: The purpose of this work was to develop, validate, and compare a highly computer-aided method for the segmentation of hot lesions in head and neck 18F-FDG PET scans.
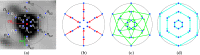
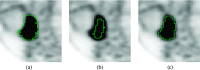
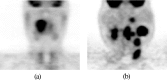
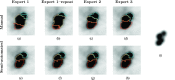
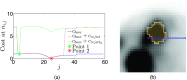
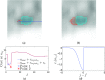
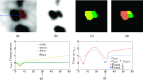
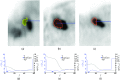
Methods: A semiautomated segmentation method was developed, which transforms the segmentation problem into a graph-based optimization problem. For this purpose, a graph structure around a user-provided approximate lesion centerpoint is constructed and a suitable cost function is derived based on local image statistics. To handle frequently occurring situations that are ambiguous (e.g., lesions adjacent to each other versus lesion with inhomogeneous uptake), several segmentation modes are introduced that adapt the behavior of the base algorithm accordingly. In addition, the authors present approaches for the efficient interactive local and global refinement of initial segmentations that are based on the "just-enough-interaction" principle. For method validation, 60 PET/CT scans from 59 different subjects with 230 head and neck lesions were utilized. All patients had squamous cell carcinoma of the head and neck. A detailed comparison with the current clinically relevant standard manual segmentation approach was performed based on 2760 segmentations produced by three experts.
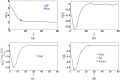
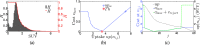
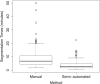
Results: Segmentation accuracy measured by the Dice coefficient of the proposed semiautomated and standard manual segmentation approach was 0.766 and 0.764, respectively. This difference was not statistically significant (p = 0.2145). However, the intra- and interoperator standard deviations were significantly lower for the semiautomated method. In addition, the proposed method was found to be significantly faster and resulted in significantly higher intra- and interoperator segmentation agreement when compared to the manual segmentation approach.
Conclusions: Lack of consistency in tumor definition is a critical barrier for radiation treatment targeting as well as for response assessment in clinical trials and in clinical oncology decision-making. The properties of the authors approach make it well suited for applications in image-guided radiation oncology, response assessment, or treatment outcome prediction.
Figures







References
-
- Fletcher J. W., Djulbegovic B., Soares H. P., Siegel B. A., Lowe V. J., Lyman G. H., Coleman R. E., Wahl R., Paschold J. C., Avril N., Einhorn L. H., Suh W. W., Samson D., Delbeke D., Gorman M., and Shields A. F., “Recommendations on the use of 18F-FDG PET in oncology,” J. Nucl. Med. 49, 480–508 (2008).10.2967/jnumed.107.047787 - DOI - PubMed
-
- Edge S. B. and A. J. C. on Cancer, AJCC Cancer Staging Manual, 7th ed. (Springer, New York, 2010), pp. xiv, 648 p.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

