Elevation of serum sphingosine-1-phosphate attenuates impaired cardiac function in experimental sepsis
- PMID: 27277195
- PMCID: PMC4899780
- DOI: 10.1038/srep27594
Elevation of serum sphingosine-1-phosphate attenuates impaired cardiac function in experimental sepsis
Abstract
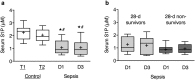
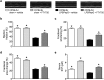
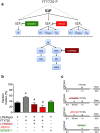
Serum levels of the lipid mediator sphingosine-1-phosphate (S1P) are reduced in septic patients and are inversely associated with disease severity. We show that serum S1P is reduced in human sepsis and in murine models of sepsis. We then investigated whether pharmacological or genetic approaches that alter serum S1P may attenuate cardiac dysfunction and whether S1P signaling might serve as a novel theragnostic tool in sepsis. Mice were challenged with lipopolysaccharide and peptidoglycan (LPS/PepG). LPS/PepG resulted in an impaired systolic contractility and reduced serum S1P. Administration of the immunomodulator FTY720 increased serum S1P, improved impaired systolic contractility and activated the phosphoinositide 3-kinase (PI3K)-pathway in the heart. Cardioprotective effects of FTY720 were abolished following administration of a S1P receptor 2 (S1P2) antagonist or a PI3K inhibitor. Sphingosine kinase-2 deficient mice had higher endogenous S1P levels and the LPS/PepG-induced impaired systolic contractility was attenuated in comparison with wild-type mice. Cardioprotective effects of FTY720 were confirmed in polymicrobial sepsis. We show here for the first time that the impaired left ventricular systolic contractility in experimental sepsis is attenuated by FTY720. Mechanistically, our results indicate that activation of S1P2 by increased serum S1P and the subsequent activation of the PI3K-Akt survival pathway significantly contributes to the observed cardioprotective effect of FTY720.
Figures





References
-
- Zaky A., Deem S., Bendjelid K. & Treggiari M. M. Characterization of cardiac dysfunction in sepsis: an ongoing challenge. Shock 41 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

