Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons
- PMID: 27277807
- PMCID: PMC4899529
- DOI: 10.1523/JNEUROSCI.0245-16.2016
Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons
Abstract
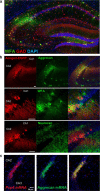
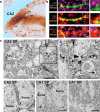
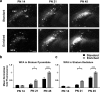
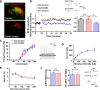
Long-term potentiation of excitatory synapses on pyramidal neurons in the stratum radiatum rarely occurs in hippocampal area CA2. Here, we present evidence that perineuronal nets (PNNs), a specialized extracellular matrix typically localized around inhibitory neurons, also surround mouse CA2 pyramidal neurons and envelop their excitatory synapses. CA2 pyramidal neurons express mRNA transcripts for the major PNN component aggrecan, identifying these neurons as a novel source for PNNs in the hippocampus. We also found that disruption of PNNs allows synaptic potentiation of normally plasticity-resistant excitatory CA2 synapses; thus, PNNs play a role in restricting synaptic plasticity in area CA2. Finally, we found that postnatal development of PNNs on CA2 pyramidal neurons is modified by early-life enrichment, suggesting that the development of circuits containing CA2 excitatory synapses are sensitive to manipulations of the rearing environment.
Significance statement: Perineuronal nets (PNNs) are thought to play a major role in restricting synaptic plasticity during postnatal development, and are altered in several models of neurodevelopmental disorders, such as schizophrenia and Rett syndrome. Although PNNs have been predominantly studied in association with inhibitory neurons throughout the brain, we describe a dense expression of PNNs around excitatory pyramidal neurons in hippocampal area CA2. We also provide insight into a previously unrecognized role for PNNs in restricting plasticity at excitatory synapses and raise the possibility of an early critical period of hippocampal plasticity that may ultimately reveal a key mechanism underlying learning and memory impairments of PNN-associated neurodevelopmental disorders.
Keywords: critical period; extracellular matrix; hippocampus; long-term potentiation.
Copyright © 2016 the authors 0270-6474/16/366312-09$15.00/0.
Figures
References
-
- Baroncelli L, Scali M, Sansevero G, Olimpico F, Manno I, Costa M, Sale A. Experience affects critical period plasticity in the visual cortex through an epigenetic regulation of histone post-translational modifications. J Neurosci. 2016;36:3430–3440. doi: 10.1523/JNEUROSCI.1787-15.2016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
