Pharmacological exploitation of the phenothiazine antipsychotics to develop novel antitumor agents-A drug repurposing strategy
- PMID: 27277973
- PMCID: PMC4899727
- DOI: 10.1038/srep27540
Pharmacological exploitation of the phenothiazine antipsychotics to develop novel antitumor agents-A drug repurposing strategy
Abstract
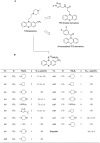
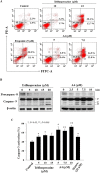
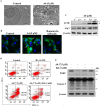
Phenothiazines (PTZs) have been used for the antipsychotic drugs for centuries. However, some of these PTZs have been reported to exhibit antitumor effects by targeting various signaling pathways in vitro and in vivo. Thus, this study was aimed at exploiting trifluoperazine, one of PTZs, to develop potent antitumor agents. This effort culminated in A4 [10-(3-(piperazin-1-yl)propyl)-2-(trifluoromethyl)-10H-phenothiazine] which exhibited multi-fold higher apoptosis-inducing activity than the parent compound in oral cancer cells. Compared to trifluoperazine, A4 demonstrated similar regulation on the phosphorylation or expression of multiple molecular targets including Akt, p38, and ERK. In addition, A4 induced autophagy, as evidenced by increased expression of the autophagy biomarkers LC3B-II and Atg5, and autophagosomes formation. The antitumor activity of A4 also related to production of reactive oxygen species and adenosine monophosphate-activated protein kinase. Importantly, the antitumor utility of A4 was extended in vivo as it, administrated at 10 and 20 mg/kg intraperitoneally, suppressed the growth of Ca922 xenograft tumors. In conclusion, the ability of A4 to target diverse aspects of cancer cell growth suggests its value in oral cancer therapy.
Figures





References
-
- Belcher R., Hayes K., Fedewa S. & Chen A. Y. Current treatment of head and neck squamous cell cancer. J Surg Oncol 110, 551–574 (2014). - PubMed
-
- Ohlow M. J. & Moosmann B. Phenothiazine: the seven lives of pharmacology’s first lead structure. Drug Discov Today 16, 119–131 (2011). - PubMed
-
- Jaszczyszyn A. et al. Chemical structure of phenothiazines and their biological activity. Pharmacol Rep 64, 16–23 (2012). - PubMed
-
- Sudeshna G. & Parimal K. Multiple non-psychiatric effects of phenothiazines: a review. Eur J Pharmacol 648, 6–14 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

