Incomplete radiofrequency ablation accelerates proliferation and angiogenesis of residual lung carcinomas via HSP70/HIF-1α
- PMID: 27278081
- PMCID: PMC4933553
- DOI: 10.3892/or.2016.4858
Incomplete radiofrequency ablation accelerates proliferation and angiogenesis of residual lung carcinomas via HSP70/HIF-1α
Abstract
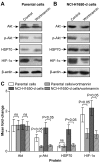
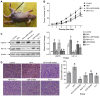
Radiofrequency ablation (RFA) therapy has been proved effective and feasible for lung cancer. However, the molecular mechanisms of local lung cancer recurrence following RFA are poorly understood. The present study aimed to evaluate the ability of HSP70/HIF-1α to affect the proliferation and angiogenesis of non-small cell lung cancers (NSCLCs) following insufficient RFA to uncover the molecular mechanisms of local recurrence. In vitro heat treatment was used to establish sublines of NCI-H1650 cells. The NCI-H1650 subline that was established by heat treatment at 54˚C had a relatively higher viability and significantly elevated heat tolerance (compared to the parental strain). After treatment with the HSP70 inhibitor VER-155008, the HIF-1α inhibitor YC-1 and PI3K/Akt inhibitor wortmannin, the viability and proliferation rate of the cells was measured. At the same time, HSP70, HIF-1α and Akt were detected by real-time PCR and western blotting. In vivo xenograft tumors were created by subcutaneously inoculating nude mice with NCI-H1650 cells. HSP70, HIF-1α and Akt were detected by western blotting, and CD34 expression was detected by immunohistochemistry before and after RFA or treatment with the VER-155008, YC-1 or wortmannin inhibitors. The heat-adapted NCI-H1650 subline established in vitro had a higher viability and proliferative activity compared to parental cells. Inhibiting HSP70/HIF-1α abolished this difference. Blocking the PI3K/Akt signaling pathway decreased HSP70/HIF-1α expression levels. In vivo, we found that incomplete RFA treatment promoted HSP70/HIF-1α and CD34 expression. Additionally, the combination of RFA and treatment targeting HSP70/HIF-1α resulted in a synergistic reduction in tumor growth compared to incomplete RFA alone. The PI3K/Akt signaling pathway is also involved in regulating HSP70/HIF-1α expression during this process. We conclude that the accelerated proliferation and angiogenesis potential of residual lung carcinomas following RFA treatment was induced by HSP70/HIF-1α, expression of which is regulated by the PI3K/Akt signaling pathway.
Figures




Similar articles
-
Hyperthermia induced HIF-1a expression of lung cancer through AKT and ERK signaling pathways.J Exp Clin Cancer Res. 2016 Jul 26;35(1):119. doi: 10.1186/s13046-016-0399-7. J Exp Clin Cancer Res. 2016. PMID: 27456341 Free PMC article.
-
Insufficient radiofrequency ablation promotes the growth of non-small cell lung cancer cells through PI3K/Akt/HIF-1α signals.Acta Biochim Biophys Sin (Shanghai). 2016 Apr;48(4):371-7. doi: 10.1093/abbs/gmw005. Epub 2016 Feb 27. Acta Biochim Biophys Sin (Shanghai). 2016. PMID: 26922319 Free PMC article.
-
Sorafenib suppresses the rapid progress of hepatocellular carcinoma after insufficient radiofrequency ablation therapy: an experiment in vivo.Acta Radiol. 2013 Mar 1;54(2):199-204. doi: 10.1258/ar.2012.120249. Epub 2012 Nov 21. Acta Radiol. 2013. PMID: 23171528
-
Targeting HIF-1α signaling pathway for gastric cancer treatment.Pharmazie. 2019 Jan 1;74(1):3-7. doi: 10.1691/ph.2019.8674. Pharmazie. 2019. PMID: 30782242 Review.
-
The Mechanism of Trivalent Inorganic Arsenic on HIF-1α: a Systematic Review and Meta-analysis.Biol Trace Elem Res. 2020 Dec;198(2):449-463. doi: 10.1007/s12011-020-02087-x. Epub 2020 Mar 2. Biol Trace Elem Res. 2020. PMID: 32124230
Cited by
-
Modelling of combination therapy using implantable anticancer drug delivery with thermal ablation in solid tumor.Sci Rep. 2020 Nov 9;10(1):19366. doi: 10.1038/s41598-020-76123-0. Sci Rep. 2020. PMID: 33168846 Free PMC article.
-
Investigation of the efficacy and safety of cryoablation and intra-arterial PD-1 inhibitor in patients with advanced disease not responding to checkpoint inhibitors: An exploratory study.Front Immunol. 2022 Sep 23;13:990224. doi: 10.3389/fimmu.2022.990224. eCollection 2022. Front Immunol. 2022. PMID: 36211329 Free PMC article.
-
Independent prognostic value of HIF-1α expression in radiofrequency ablation of lung cancer.Oncol Lett. 2020 Jan;19(1):849-857. doi: 10.3892/ol.2019.11130. Epub 2019 Nov 21. Oncol Lett. 2020. PMID: 31897199 Free PMC article.
-
Roles of hypoxia-inducible factor in hepatocellular carcinoma under local ablation therapies.Front Pharmacol. 2023 Feb 6;14:1086813. doi: 10.3389/fphar.2023.1086813. eCollection 2023. Front Pharmacol. 2023. PMID: 36814489 Free PMC article. Review.
-
Crosstalk between microwave ablation and ferroptosis: The next hot topic?Front Oncol. 2023 Jan 13;13:1099731. doi: 10.3389/fonc.2023.1099731. eCollection 2023. Front Oncol. 2023. PMID: 36712497 Free PMC article. Review.
References
-
- Chen WQ, Zhang SW, Zou XN, Zhao P. An analysis of lung cancer mortality in China, 2004–2005. Zhonghua Yu Fang Yi Xue Za Zhi. 2010;44:378–382. In Chinese. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

