The function and regulation of acid-sensing ion channels (ASICs) and the epithelial Na(+) channel (ENaC): IUPHAR Review 19
- PMID: 27278329
- PMCID: PMC4995293
- DOI: 10.1111/bph.13533
The function and regulation of acid-sensing ion channels (ASICs) and the epithelial Na(+) channel (ENaC): IUPHAR Review 19
Abstract
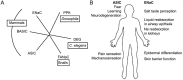
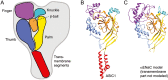
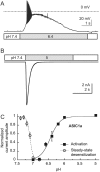
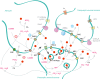
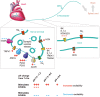
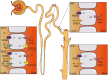
Acid-sensing ion channels (ASICs) and the epithelial Na(+) channel (ENaC) are both members of the ENaC/degenerin family of amiloride-sensitive Na(+) channels. ASICs act as proton sensors in the nervous system where they contribute, besides other roles, to fear behaviour, learning and pain sensation. ENaC mediates Na(+) reabsorption across epithelia of the distal kidney and colon and of the airways. ENaC is a clinically used drug target in the context of hypertension and cystic fibrosis, while ASIC is an interesting potential target. Following a brief introduction, here we will review selected aspects of ASIC and ENaC function. We discuss the origin and nature of pH changes in the brain and the involvement of ASICs in synaptic signalling. We expose how in the peripheral nervous system, ASICs cover together with other ion channels a wide pH range as proton sensors. We introduce the mechanisms of aldosterone-dependent ENaC regulation and the evidence for an aldosterone-independent control of ENaC activity, such as regulation by dietary K(+) . We then provide an overview of the regulation of ENaC by proteases, a topic of increasing interest over the past few years. In spite of the profound differences in the physiological and pathological roles of ASICs and ENaC, these channels share many basic functional and structural properties. It is likely that further research will identify physiological contexts in which ASICs and ENaC have similar or overlapping roles.
© 2016 The British Pharmacological Society.
Figures
References
-
- Abdrakhmanova G, Cleemann L, Lindstrom J, Morad M (2004). Differential modulation of beta2 and beta4 subunits of human neuronal nicotinic acetylcholine receptors by acidification. Mol Pharmacol 66: 347–355. - PubMed
-
- Abdrakhmanova G, Dorfman J, Xiao Y, Morad M (2002). Protons enhance the gating kinetics of the alpha3/beta4 neuronal nicotinic acetylcholine receptor by increasing its apparent affinity to agonists. Mol Pharmacol 61: 369–378. - PubMed
-
- Adams CM, Snyder PM, Welsh MJ (1999). Paradoxical stimulation of a DEG/ENaC channel by amiloride. J Biol Chem 274: 15500–15504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

