JAK3-STAT pathway blocking benefits in experimental lupus nephritis
- PMID: 27278657
- PMCID: PMC4898357
- DOI: 10.1186/s13075-016-1034-x
JAK3-STAT pathway blocking benefits in experimental lupus nephritis
Erratum in
-
Erratum to: JAK3-STAT pathway blocking benefits in experimental lupus nephritis.Arthritis Res Ther. 2016 Jul 1;18(1):152. doi: 10.1186/s13075-016-1058-2. Arthritis Res Ther. 2016. PMID: 27368974 Free PMC article. No abstract available.
Abstract
Background: Lupus nephritis (LN) is a complex chronic autoimmune disease of unknown etiology characterized by loss of tolerance against several self-antigens. Cytokines are known to be central players in LN pathogenesis. The Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway is one important pathway that mediates signal transduction of several cytokines. In this study, we examined the pathogenic role of this pathway and how CP-690,550 treatment influences LN outcome.
Methods: Six-month-old NZB/NZWF1 mice were divided into two different treatment groups: (1) control animals given vehicle treatment, cyclophosphamide, and mycophenolate mofetil treatment as positive controls of the therapy and (2) mice treated with CP-690,550, a JAK3 inhibitor. Mice were treated for 12 weeks. We evaluated renal function, anti-double-stranded DNA (anti-dsDNA) antibody, renal histology changes, kidney complement and immunoglobulin G (IgG) deposits, T-cell and macrophage infiltration, kidney inflammatory gene expression, and circulating cytokine changes.
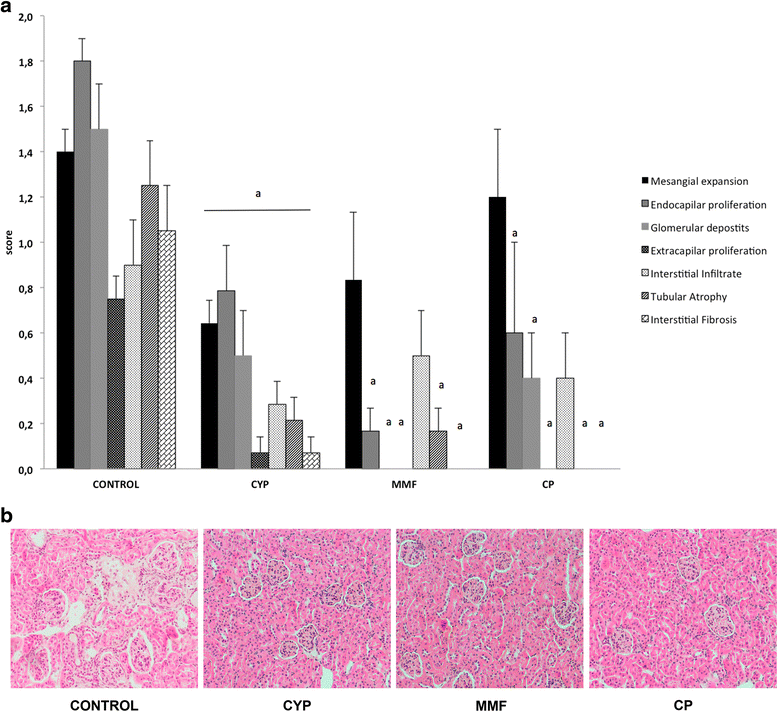
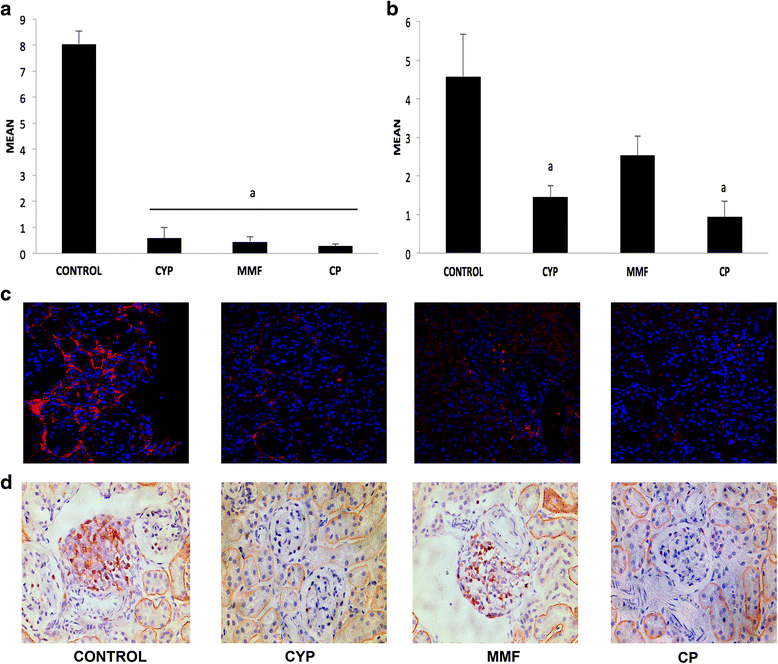
Results: CP-690,550 treatment significantly reduced proteinuria and improved renal function and histological lesions of the kidney. Compared with vehicle-treated animals, those undergoing CP-690,550 treatment showed significantly diminished anti-dsDNA antibody and complement component C3 and IgG deposition in glomeruli. We also observed a significant reduction of T-cell and macrophage infiltration. Kidney gene expression revealed a reduction in inflammatory cytokines and complement and related macrophage-attracting genes. Circulating inflammatory cytokines were also reduced with treatment.
Conclusions: On the basis of our results, we conclude that the JAK-STAT pathway is implicated in the progression of renal inflammation in NZB/WF1 mice and that targeting JAK3 with CP-690,550 is effective in slowing down the course of experimental LN. Thus, CP-690,550 could become a new therapeutic tool in LN and other autoimmune diseases.
Keywords: Autoimmunity; Glomerulonephritis; JAK3 inhibitor; Kidney infiltrate; Lupus nephritis.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

