Gender differences in murine pulmonary responses elicited by cellulose nanocrystals
- PMID: 27278671
- PMCID: PMC4898310
- DOI: 10.1186/s12989-016-0140-x
Gender differences in murine pulmonary responses elicited by cellulose nanocrystals
Abstract
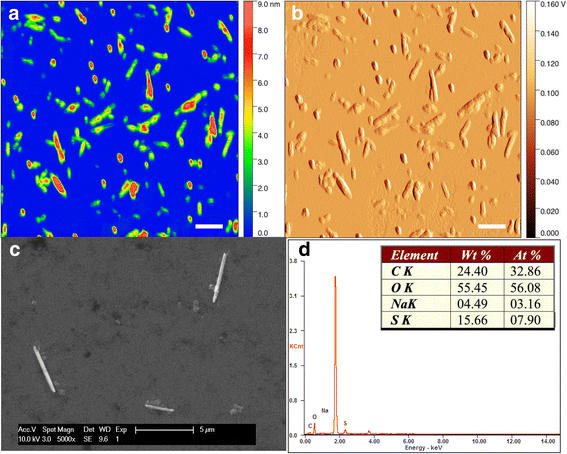
Background: Cellulose-based materials have been used for centuries to manufacture different goods derived from forestry and agricultural sources. In the growing field of nanocellulose applications, its uniquely engineered properties are instrumental for inventive products coming to competitive markets. Due to their high aspect ratio and stiffness, it is speculated that cellulose nanocrystals (CNC) may cause similar pulmonary toxicity as carbon nanotubes and asbestos, thus posing a potential negative impact on public health and the environment.
Methods: The present study was undertaken to investigate the pulmonary outcomes induced by repeated exposure to respirable CNC. C57BL/6 female and male mice were exposed by pharyngeal aspiration to CNC (40 μg/mouse) 2 times a week for 3 weeks. Several biochemical endpoints and pathophysiological outcomes along with gene expression changes were evaluated and compared in the lungs of male and female mice.
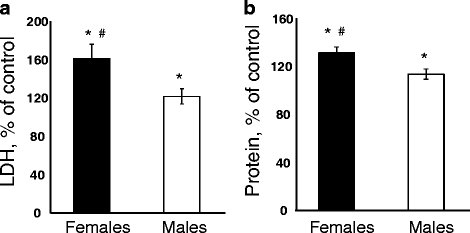
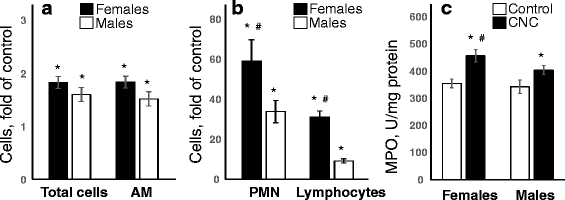
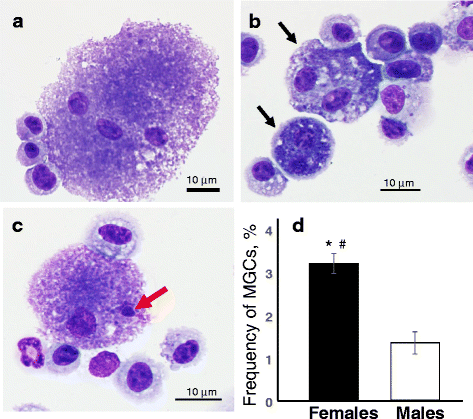
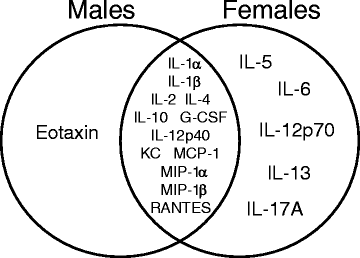
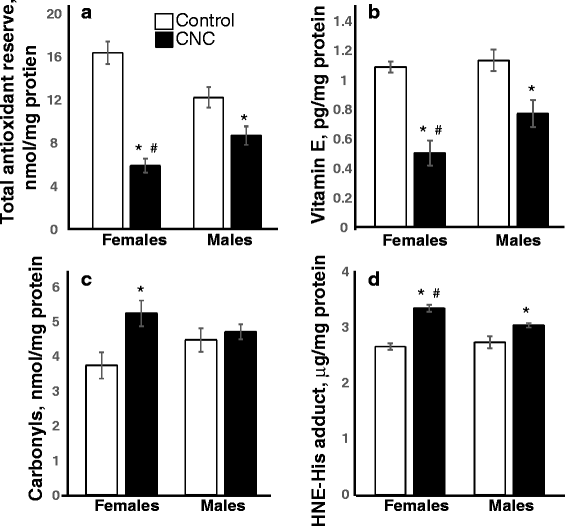
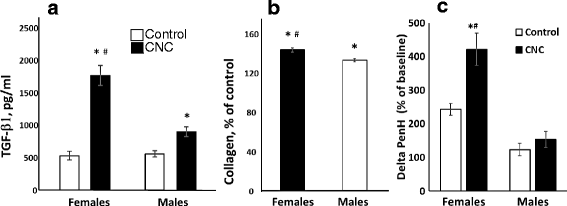
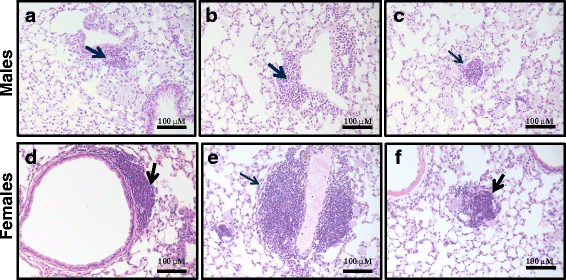
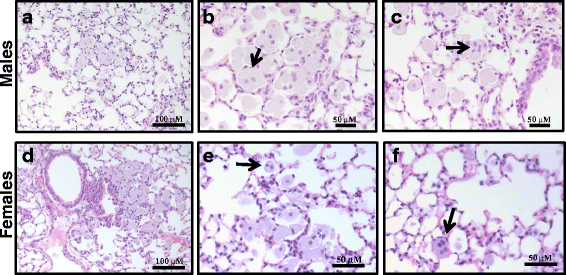
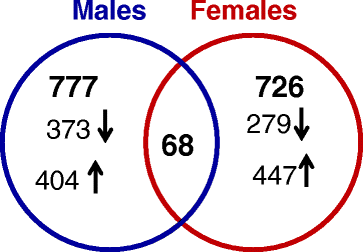
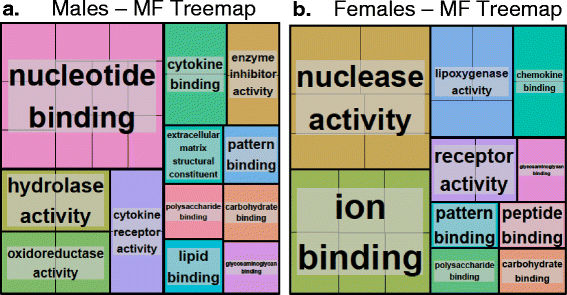
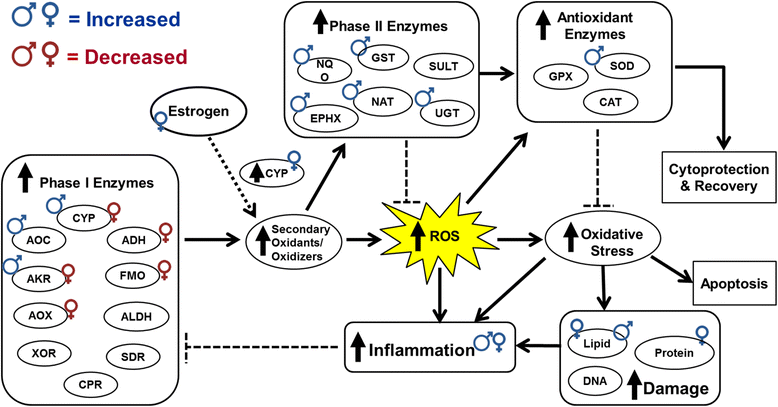
Results: Exposure to respirable CNC caused pulmonary inflammation and damage, induced oxidative stress, elevated TGF-β and collagen levels in lung, and impaired pulmonary functions. Notably, these effects were markedly more pronounced in females compared to male mice. Moreover, sex differences in responses to pulmonary exposure to CNC were also detected at the level of global mRNA expression as well as in inflammatory cytokine/chemokine activity.
Conclusions: Overall, our results indicate that there are considerable differences in responses to respirable CNC based on gender with a higher pulmonary toxicity observed in female mice.
Keywords: Cellulose; Gender differences; Inflammation; Oxidative stress; Pulmonary toxicity; mRNA expression.
Figures
Comment in
-
Comment on Shvedova et al. (2016), "gender differences in murine pulmonary responses elicited by cellulose nanocrystals".Part Fibre Toxicol. 2016 Nov 4;13(1):59. doi: 10.1186/s12989-016-0170-4. Part Fibre Toxicol. 2016. PMID: 27814761 Free PMC article.
References
-
- Postek MT, Moon RJ, Rudie A, Bilodeau M. Production and Applications of Cellulose Nanomaterials. Peachtree Corners, GA: TAPPI; 2013.
-
- Floros M, Hojabri L, Abraham E, Jose J, Thomas S, Pothan L, et al. Enhancement of thermal stability, strength and extensibility of lipid-based polyurethanes with cellulose-based nanofibers. Polym Degrad Stab. 2012;97(10):1970–8. doi: 10.1016/j.polymdegradstab.2012.02.016. - DOI
-
- H.P.S.A. K, A.H. B, A.F.I. Y. Green composites from sustainable cellulose nanofibrils: a review. Carbohyd Polym. 2012;87:963-79.
-
- Siqueira G, Bras J, Dufresne A. Cellulosic bionanocomposites: a review of preparation, properties and applications. Polym. 2010;2:728–65. doi: 10.3390/polym2040728. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

