Triclosan antimicrobial polymers
- PMID: 27280150
- PMCID: PMC4893770
- DOI: 10.3934/molsci.2016.1.88
Triclosan antimicrobial polymers
Abstract
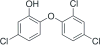
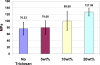
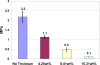
Triclosan antimicrobial molecular fluctuating energies of nonbonding electron pairs for the oxygen atom by ether bond rotations are reviewed with conformational computational chemistry analyses. Subsequent understanding of triclosan alternating ether bond rotations is able to help explain several material properties in Polymer Science. Unique bond rotation entanglements between triclosan and the polymer chains increase both the mechanical properties of polymer toughness and strength that are enhanced even better through secondary bonding relationships. Further, polymer blend compatibilization is considered due to similar molecular relationships and polarities. With compatibilization of triclosan in polymers a more uniform stability for nonpolar triclosan in the polymer solid state is retained by the antimicrobial for extremely low release with minimum solubility into aqueous solution. As a result, triclosan is projected for long extended lifetimes as an antimicrobial polymer additive. Further, triclosan rapid alternating ether bond rotations disrupt secondary bonding between chain monomers in the resin state to reduce viscosity and enhance polymer blending. Thus, triclosan is considered for a polymer additive with multiple properties to be an antimicrobial with additional benefits as a nonpolar toughening agent and a hydrophobic wetting agent. The triclosan material relationships with alternating ether bond rotations are described through a complete different form of medium by comparisons with known antimicrobial properties that upset bacterial cell membranes through rapid fluctuating mechanomolecular energies. Also, triclosan bond entanglements with secondary bonding can produce structural defects in weak bacterial lipid membranes requiring pliability that can then interfere with cell division. Regarding applications with polymers, triclosan can be incorporated by mixing into a resin system before cure, melt mixed with thermoplastic polymers that set on cooling into a solid or alternatively applied as a coating through several different methods with dissolving into an organic solvent and dried on by evaporation as a common means.
Keywords: Antimicrobial; bond entanglements; bond rotation; computational chemistry; mechanomolecular; polymer; secondary bonding; strength; toughness; viscosity.
Conflict of interest statement
The author declares no conflicts of interest in this paper.
Figures

Similar articles
-
Triclosan Computational Conformational Chemistry Analysis for Antimicrobial Properties in Polymers.J Nat Sci. 2015 Mar;1(3):e54. J Nat Sci. 2015. PMID: 25879080 Free PMC article.
-
Computational conformational antimicrobial analysis developing mechanomolecular theory for polymer biomaterials in materials science and engineering.Int J Comput Mater Sci Eng. 2014 Mar;3(1):1450003. doi: 10.1142/S2047684114500031. Int J Comput Mater Sci Eng. 2014. PMID: 25598972 Free PMC article.
-
Antibacterial efficacy of triclosan-incorporated polymers.Am J Infect Control. 2001 Apr;29(2):124-5. doi: 10.1067/mic.2001.113229. Am J Infect Control. 2001. PMID: 11287882
-
Hydrogen-Bond-Mediated Polymers: Strengthening, Toughening, and Stabilizing Effects.Chemistry. 2025 May 14;31(27):e202500674. doi: 10.1002/chem.202500674. Epub 2025 Apr 16. Chemistry. 2025. PMID: 40167175 Review.
-
Dentifrices containing new agents for the control of plaque and gingivitis: microbiological aspects.J Clin Periodontol. 1991 Jul;18(6):462-7. doi: 10.1111/j.1600-051x.1991.tb02317.x. J Clin Periodontol. 1991. PMID: 1890229 Review.
Cited by
-
Ten questions concerning the implications of carpet on indoor chemistry and microbiology.Build Environ. 2019 Dec 18;170:1-16. doi: 10.1016/j.buildenv.2019.106589. Build Environ. 2019. PMID: 32055099 Free PMC article.
-
Design and Characterization of PAA/CHI/Triclosan Multilayer Films with Long-Term Antibacterial Activity.Polymers (Basel). 2025 Jun 27;17(13):1789. doi: 10.3390/polym17131789. Polymers (Basel). 2025. PMID: 40647799 Free PMC article.
-
Recent Advances in Antimicrobial Coatings and Material Modification Strategies for Preventing Urinary Catheter-Associated Complications.Biomedicines. 2022 Oct 14;10(10):2580. doi: 10.3390/biomedicines10102580. Biomedicines. 2022. PMID: 36289841 Free PMC article. Review.
-
Triclosan: A Small Molecule with Controversial Roles.Antibiotics (Basel). 2022 May 30;11(6):735. doi: 10.3390/antibiotics11060735. Antibiotics (Basel). 2022. PMID: 35740142 Free PMC article. Review.
-
Plastics in municipal drinking water and wastewater treatment plant effluents: challenges and opportunities for South Africa-a review.Environ Sci Pollut Res Int. 2020 Apr;27(12):12953-12966. doi: 10.1007/s11356-020-08194-5. Epub 2020 Mar 2. Environ Sci Pollut Res Int. 2020. PMID: 32124288 Review.
References
-
-
Ciba Specialty Chemicals (2001) Antimicrobials Irgasan DP 300 Irgacare MP Irgacide LP 10. General information on chemical, physical and microbiological properties. Brochure 2520 Ciba Specialty Chemical Corporation, High Point, NC 27265Pub. No. AgB2520e.02.2001. Edited in Switzerland.
-
-
- European Commission, Scientific Committees on Consumer Safety (SCCS) Opinion on Triclosan, Antimicrobial Resistance. (SCCP/1251/09).Directorate-General for Health and Consumers, Opinion approved 7th Plenary. 2010 Available from: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sc....
-
- Australian Government, Department of Health and Ageing NICNAS. (Priority Existing Chemical Assessment Report No. 30, Triclosan).National Industrial Chemical Notification and Assessment Scheme, Sydney Australia. 2009 Available from: http://www.nicnas.gov.au/publications/car/pec/pec30/pec_30_full_report_p....
-
- Alberts B, Bray D, Lewis J, et al. Molecular Biology of the Cell. 3. New York: Garland Publishing, Inc; 1994. Small molecules, energy and biosynthesis; pp. 45–55.
-
- Brown WH, Foote CS, Everson BL, et al. Organic Chemistry. 5. Belmont, CA: Brooks/Cole; 2009.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
