Isolation and Reactivity of Trifluoromethyl Iodonium Salts
- PMID: 27280169
- PMCID: PMC4882740
- DOI: 10.1021/acscentsci.6b00119
Isolation and Reactivity of Trifluoromethyl Iodonium Salts
Abstract
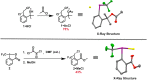
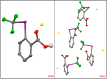
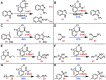
The strategic incorporation of the trifluoromethyl (CF3) functionality within therapeutic or agrochemical agents is a proven strategy for altering their associated physicochemical properties (e.g., metabolic stability, lipophilicity, and bioavailability). Electrophilic trifluoromethylation has emerged as an important methodology for installing the CF3 moiety onto an array of molecular architectures, and, in particular, CF3 λ(3)-iodanes have garnered significant interest because of their unique reactivity and ease of handling. Trifluoromethylations mediated by these hypervalent iodine reagents often require activation through an exogenous Lewis or Brønsted acid; thus, putative intermediates invoked in these transformations are cationic CF3 iodoniums. These iodoniums have, thus far, eluded isolation and investigation of their innate reactivity (which has encouraged speculation that such species cannot be accessed). A more complete understanding of the mechanistic relevance of CF3 iodoniums is paramount for the development of new trifluoromethylative strategies involving λ(3)-iodanes. Here, we demonstrate that CF3 iodonium salts are readily prepared from common λ(3)-iodane precursors and exhibit remarkable persistence under ambient conditions. These reagents are competent electrophiles for a variety of trifluoromethylation reactions, and their reactivity is reminiscent of that observed when CF3 iodanes are activated using Lewis acids. As such, our results suggest the mechanistic relevance of CF3 iodonium intermediates in trifluoromethylative processes mediated by λ(3)-iodanes. The isolation of CF3 iodonium salts also presents the unique opportunity to employ them more generally as mechanistic probes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

