Quantitative podocyte parameters predict human native kidney and allograft half-lives
- PMID: 27280173
- PMCID: PMC4894348
- DOI: 10.1172/jci.insight.86943
Quantitative podocyte parameters predict human native kidney and allograft half-lives
Abstract
Background: Kidney function decreases with age. A potential mechanistic explanation for kidney and allograft half-life has evolved through the realization that linear reduction in glomerular podocyte density could drive progressive glomerulosclerosis to impact both native kidney and allograft half-lives.
Methods: Predictions from podometrics (quantitation of podocyte parameters) were tested using independent pathologic, functional, and outcome data for native kidneys and allografts derived from published reports and large registries.
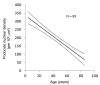
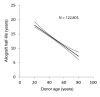
Results: With age, native kidneys exponentially develop glomerulosclerosis, reduced renal function, and end-stage kidney disease, projecting a finite average kidney life span. The slope of allograft failure rate versus age parallels that of reduction in podocyte density versus age. Quantitative modeling projects allograft half-life at any donor age, and rate of podocyte detachment parallels the observed allograft loss rate.
Conclusion: Native kidneys are designed to have a limited average life span of about 100-140 years. Allografts undergo an accelerated aging-like process that accounts for their unexpectedly short half-life (about 15 years), the observation that older donor age is associated with shorter allograft half-life, and the fact that long-term allograft survival has not substantially improved. Podometrics provides potential readouts for these processes, thereby offering new approaches for monitoring and intervention.
Funding: National Institutes of Health.
Figures



Similar articles
-
Accelerated podocyte detachment early after kidney transplantation is related to long-term allograft loss of function.Nephrol Dial Transplant. 2019 Jul 1;34(7):1232-1239. doi: 10.1093/ndt/gfy350. Nephrol Dial Transplant. 2019. PMID: 30500951 Free PMC article.
-
The two kidney to one kidney transition and transplant glomerulopathy: a podocyte perspective.J Am Soc Nephrol. 2015 Jun;26(6):1450-65. doi: 10.1681/ASN.2014030287. Epub 2014 Nov 11. J Am Soc Nephrol. 2015. PMID: 25388223 Free PMC article.
-
Podometrics in Japanese Living Donor Kidneys: Associations with Nephron Number, Age, and Hypertension.J Am Soc Nephrol. 2021 May 3;32(5):1187-1199. doi: 10.1681/ASN.2020101486. Epub 2021 Feb 24. J Am Soc Nephrol. 2021. PMID: 33627345 Free PMC article.
-
Urinary podocyte markers of disease activity, therapeutic efficacy, and long-term outcomes in acute and chronic kidney diseases.Clin Exp Nephrol. 2024 Jun;28(6):496-504. doi: 10.1007/s10157-024-02465-y. Epub 2024 Feb 25. Clin Exp Nephrol. 2024. PMID: 38402504 Free PMC article. Review.
-
Podometrics as a Potential Clinical Tool for Glomerular Disease Management.Semin Nephrol. 2015 May;35(3):245-55. doi: 10.1016/j.semnephrol.2015.04.004. Semin Nephrol. 2015. PMID: 26215862 Free PMC article. Review.
Cited by
-
Nephrons, podocytes and chronic kidney disease: Strategic antihypertensive therapy for renoprotection.Hypertens Res. 2023 Feb;46(2):299-310. doi: 10.1038/s41440-022-01061-5. Epub 2022 Oct 12. Hypertens Res. 2023. PMID: 36224286 Free PMC article. Review.
-
Accelerated podocyte detachment and progressive podocyte loss from glomeruli with age in Alport Syndrome.Kidney Int. 2017 Dec;92(6):1515-1525. doi: 10.1016/j.kint.2017.05.017. Epub 2017 Jul 26. Kidney Int. 2017. PMID: 28754557 Free PMC article.
-
Recognition of intraglomerular histological features with deep learning in protocol transplant biopsies and their association with kidney function and prognosis.Clin Kidney J. 2024 Feb 2;17(2):sfae019. doi: 10.1093/ckj/sfae019. eCollection 2024 Feb. Clin Kidney J. 2024. PMID: 38370429 Free PMC article.
-
Advancing the application of the analytical renal pathology system in allograft IgA nephropathy patients.Ren Fail. 2024 Dec;46(1):2322043. doi: 10.1080/0886022X.2024.2322043. Epub 2024 Feb 29. Ren Fail. 2024. PMID: 38425049 Free PMC article.
-
The growth hormone/IGF-1 axis is a risk factor for long-term kidney allograft failure.JCI Insight. 2025 May 6;10(11):e188485. doi: 10.1172/jci.insight.188485. eCollection 2025 Jun 9. JCI Insight. 2025. PMID: 40327402 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

