Eradication of Acute Myeloid Leukemia with FLT3 Ligand-Targeted miR-150 Nanoparticles
- PMID: 27280396
- PMCID: PMC4970973
- DOI: 10.1158/0008-5472.CAN-15-2949
Eradication of Acute Myeloid Leukemia with FLT3 Ligand-Targeted miR-150 Nanoparticles
Abstract
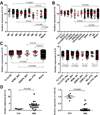
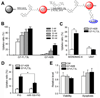
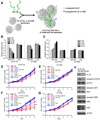
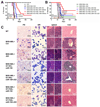
Acute myeloid leukemia (AML) is a common and fatal form of hematopoietic malignancy. Overexpression and/or mutations of FLT3 have been shown to occur in the majority of cases of AML. Our analysis of a large-scale AML patient cohort (N = 562) indicates that FLT3 is particularly highly expressed in some subtypes of AML, such as AML with t(11q23)/MLL-rearrangements or FLT3-ITD. Such AML subtypes are known to be associated with unfavorable prognosis. To treat FLT3-overexpressing AML, we developed a novel targeted nanoparticle system: FLT3 ligand (FLT3L)-conjugated G7 poly(amidoamine) (PAMAM) nanosized dendriplex encapsulating miR-150, a pivotal tumor suppressor and negative regulator of FLT3 We show that the FLT3L-guided miR-150 nanoparticles selectively and efficiently target FLT3-overexpressing AML cells and significantly inhibit viability/growth and promote apoptosis of the AML cells. Our proof-of-concept animal model studies demonstrate that the FLT3L-guided miR-150 nanoparticles tend to concentrate in bone marrow, and significantly inhibit progression of FLT3-overexpressing AML in vivo, while exhibiting no obvious side effects on normal hematopoiesis. Collectively, we have developed a novel targeted therapeutic strategy, using FLT3L-guided miR-150-based nanoparticles, to treat FLT3-overexpressing AML with high efficacy and minimal side effects. Cancer Res; 76(15); 4470-80. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Figures

References
-
- Dohner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373:1136–1152. - PubMed
-
- Lyman SD, James L, Vanden Bos T, de Vries P, Brasel K, Gliniak B, et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993;75:1157–1167. - PubMed
-
- Bullinger L, Dohner K, Kranz R, Stirner C, Frohling S, Scholl C, et al. An FLT3 gene-expression signature predicts clinical outcome in normal karyotype AML. Blood. 2008;111:4490–4495. - PubMed
-
- Whitman SP, Maharry K, Radmacher MD, Becker H, Mrozek K, Margeson D, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:3622–3626. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

