Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy
- PMID: 27280428
- PMCID: PMC5142222
- DOI: 10.1152/ajpregu.00137.2016
Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy
Abstract
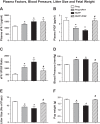
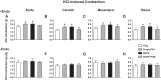
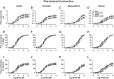
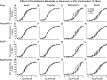
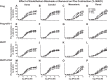
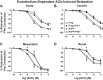
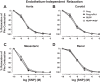
Preeclampsia (PE) is a pregnancy-related hypertensive disorder (HTN-Preg) with unclear mechanism. An imbalance between antiangiogenic soluble fms-like tyrosine kinase-1 (sFlt-1) and angiogenic placental growth factor (PlGF) has been observed in PE, but the vascular targets and signaling pathways involved are unclear. We assessed the extent of sFlt-1/PlGF imbalance and vascular dysfunction in a rat model of HTN-Preg produced by reduction of uteroplacental perfusion pressure (RUPP), and tested whether inducing a comparable sFlt-1/PlGF imbalance by infusing sFlt-1 (10 μg·kg(-1)·day(-1)) in day 14 pregnant (Preg) rats cause similar increases in blood pressure (BP) and vascular reactivity. Using these guiding measurements, we then tested whether restoring sFlt-1/PlGF balance by infusing PIGF (20 μg·kg(-1)·day(-1)) in RUPP rats would improve BP and vascular function. On gestational day 19, BP was in Preg+sFlt-1 and RUPP > Preg, and in RUPP+PlGF < RUPP rats. Plasma sFlt-1/PlGF ratio was increased in Preg+sFlt-1, and RUPP and was reduced in RUPP+PlGF rats. In isolated endothelium-intact aorta, carotid, mesenteric, and renal artery, phenylephrine (Phe)- and high KCl-induced contraction was in Preg+sFlt-1 and RUPP > Preg, and in RUPP+PlGF < RUPP. The differences in vascular reactivity to Phe and KCl between groups were less apparent in vessels treated with the nitric oxide synthase (NOS) inhibitor l-NAME or guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) or endothelium-denuded, suggesting changes in endothelial NO-cGMP pathway. In Phe precontracted vessels, ACh-induced relaxation was in Preg+sFlt-1 and RUPP < Preg, and in RUPP+PlGF > RUPP, and was blocked by N(ω)-nitro-l-arginine methyl ester (l-NAME) or ODQ treatment or endothelium removal. Western blots revealed that aortic total endothelial NOS (eNOS) and activated phosphorylated-eNOS were in Preg+sFlt-1 and RUPP < Preg and in RUPP+PlGF > RUPP. ACh-induced vascular nitrate/nitrite production was in Preg+sFlt-1 and RUPP < Preg, and in RUPP+PlGF > RUPP. Vascular relaxation to the exogenous NO donor sodium nitroprusside was not different among groups. Thus, a tilt in the angiogenic balance toward anti-angiogenic sFlt-1 is associated with decreased vascular relaxation and increased vasoconstriction and BP. Restoring the angiogenic/antiangiogenic balance using PlGF enhances endothelial NO-cGMP vascular relaxation and decreases vasoconstriction and BP in HTN-Preg rats and could offer a new approach in the management of PE.
Keywords: angiogenesis; calcium; endothelium; hypertension; nitric oxide; preeclampsia; vascular smooth muscle.
Copyright © 2016 the American Physiological Society.
Figures



Similar articles
-
Placental growth factor reverses decreased vascular and uteroplacental MMP-2 and MMP-9 and increased MMP-1 and MMP-7 and collagen types I and IV in hypertensive pregnancy.Am J Physiol Heart Circ Physiol. 2018 Jul 1;315(1):H33-H47. doi: 10.1152/ajpheart.00045.2018. Epub 2018 Mar 23. Am J Physiol Heart Circ Physiol. 2018. PMID: 29569955 Free PMC article.
-
Angiogenic imbalance and diminished matrix metalloproteinase-2 and -9 underlie regional decreases in uteroplacental vascularization and feto-placental growth in hypertensive pregnancy.Biochem Pharmacol. 2017 Dec 15;146:101-116. doi: 10.1016/j.bcp.2017.09.005. Epub 2017 Sep 11. Biochem Pharmacol. 2017. PMID: 28912068 Free PMC article.
-
Altered matrix metalloproteinase-2 and -9 expression/activity links placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy.Biochem Pharmacol. 2014 Jun 1;89(3):370-85. doi: 10.1016/j.bcp.2014.03.017. Epub 2014 Apr 3. Biochem Pharmacol. 2014. PMID: 24704473 Free PMC article.
-
Combining Biomarkers to Predict Pregnancy Complications and Redefine Preeclampsia: The Angiogenic-Placental Syndrome.Hypertension. 2020 Apr;75(4):918-926. doi: 10.1161/HYPERTENSIONAHA.119.13763. Epub 2020 Feb 17. Hypertension. 2020. PMID: 32063058 Free PMC article. Review.
-
Regulation of vascular angiotensin II type 1 and type 2 receptor and angiotensin-(1-7)/MasR signaling in normal and hypertensive pregnancy.Biochem Pharmacol. 2024 Feb;220:115963. doi: 10.1016/j.bcp.2023.115963. Epub 2023 Dec 5. Biochem Pharmacol. 2024. PMID: 38061417 Free PMC article. Review.
Cited by
-
Reliability of Rodent and Rabbit Models in Preeclampsia Research.Int J Mol Sci. 2022 Nov 18;23(22):14344. doi: 10.3390/ijms232214344. Int J Mol Sci. 2022. PMID: 36430816 Free PMC article. Review.
-
Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia.Am J Physiol Heart Circ Physiol. 2020 Sep 1;319(3):H661-H681. doi: 10.1152/ajpheart.00202.2020. Epub 2020 Aug 7. Am J Physiol Heart Circ Physiol. 2020. PMID: 32762557 Free PMC article. Review.
-
Increased uterine arterial tone, stiffness and remodeling with augmented matrix metalloproteinase-1 and -7 in uteroplacental ischemia-induced hypertensive pregnancy.Biochem Pharmacol. 2024 Oct;228:116227. doi: 10.1016/j.bcp.2024.116227. Epub 2024 Apr 19. Biochem Pharmacol. 2024. PMID: 38643908
-
Biomarkers used in studying air pollution exposure during pregnancy and perinatal outcomes: a review.Biomarkers. 2017 Sep;22(6):489-501. doi: 10.1080/1354750X.2017.1339294. Epub 2017 Jun 21. Biomarkers. 2017. PMID: 28581828 Free PMC article. Review.
-
High-fat diet from parental generation exaggerates body and adipose tissue weights in pregnant offspring.PLoS One. 2020 Aug 20;15(8):e0237708. doi: 10.1371/journal.pone.0237708. eCollection 2020. PLoS One. 2020. PMID: 32817646 Free PMC article.
References
-
- Agunanne EE, Uddin MN, Horvat D, Puschett JB. Contribution of angiogenic factors in a rat model of pre-eclampsia. Am J Nephrol 32: 332–339, 2010. - PubMed
-
- Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol 290: R1–R10, 2006. - PubMed
-
- Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. - PubMed
-
- Avivi A, Resnick MB, Nevo E, Joel A, Levy AP. Adaptive hypoxic tolerance in the subterranean mole rat Spalax ehrenbergi: the role of vascular endothelial growth factor. FEBS Lett 452: 133–140, 1999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

