The Drosophila ETV5 Homologue Ets96B: Molecular Link between Obesity and Bipolar Disorder
- PMID: 27280443
- PMCID: PMC4900636
- DOI: 10.1371/journal.pgen.1006104
The Drosophila ETV5 Homologue Ets96B: Molecular Link between Obesity and Bipolar Disorder
Abstract
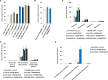
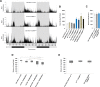
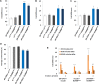
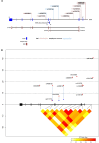
Several reports suggest obesity and bipolar disorder (BD) share some physiological and behavioural similarities. For instance, obese individuals are more impulsive and have heightened reward responsiveness, phenotypes associated with BD, while bipolar patients become obese at a higher rate and earlier age than people without BD; however, the molecular mechanisms of such an association remain obscure. Here we demonstrate, using whole transcriptome analysis, that Drosophila Ets96B, homologue of obesity-linked gene ETV5, regulates cellular systems associated with obesity and BD. Consistent with a role in obesity and BD, loss of nervous system Ets96B during development increases triacylglyceride concentration, while inducing a heightened startle-response, as well as increasing hyperactivity and reducing sleep. Of notable interest, mouse Etv5 and Drosophila Ets96B are expressed in dopaminergic-rich regions, and loss of Ets96B specifically in dopaminergic neurons recapitulates the metabolic and behavioural phenotypes. Moreover, our data indicate Ets96B inhibits dopaminergic-specific neuroprotective systems. Additionally, we reveal that multiple SNPs in human ETV5 link to body mass index (BMI) and BD, providing further evidence for ETV5 as an important and novel molecular intermediate between obesity and BD. We identify a novel molecular link between obesity and bipolar disorder. The Drosophila ETV5 homologue Ets96B regulates the expression of cellular systems with links to obesity and behaviour, including the expression of a conserved endoplasmic reticulum molecular chaperone complex known to be neuroprotective. Finally, a connection between the obesity-linked gene ETV5 and bipolar disorder emphasizes a functional relationship between obesity and BD at the molecular level.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Evidence that genes involved in hedgehog signaling are associated with both bipolar disorder and high BMI.Transl Psychiatry. 2019 Nov 21;9(1):315. doi: 10.1038/s41398-019-0652-x. Transl Psychiatry. 2019. PMID: 31754094 Free PMC article.
-
Loss of porin function in dopaminergic neurons of Drosophila is suppressed by Buffy.J Biomed Sci. 2016 Nov 24;23(1):84. doi: 10.1186/s12929-016-0300-1. J Biomed Sci. 2016. PMID: 27881168 Free PMC article.
-
Impaired endoplasmic reticulum stress response in B-lymphoblasts from patients with bipolar-I disorder.Biol Psychiatry. 2007 Jul 15;62(2):141-7. doi: 10.1016/j.biopsych.2006.10.014. Epub 2007 Jan 9. Biol Psychiatry. 2007. PMID: 17217928
-
Epigenetics and bipolar disorder: new opportunities and challenges.Am J Med Genet C Semin Med Genet. 2003 Nov 15;123C(1):65-75. doi: 10.1002/ajmg.c.20015. Am J Med Genet C Semin Med Genet. 2003. PMID: 14601038 Review.
-
Plausible therapeutic effects of melatonin and analogs in the dopamine-associated pathophysiology of bipolar disorder.J Psychiatr Res. 2025 Feb;182:13-20. doi: 10.1016/j.jpsychires.2024.12.046. Epub 2025 Jan 2. J Psychiatr Res. 2025. PMID: 39793267 Review.
Cited by
-
Evidence that genes involved in hedgehog signaling are associated with both bipolar disorder and high BMI.Transl Psychiatry. 2019 Nov 21;9(1):315. doi: 10.1038/s41398-019-0652-x. Transl Psychiatry. 2019. PMID: 31754094 Free PMC article.
-
Exposure to the environmental pollutant bisphenol A diglycidyl ether (BADGE) causes cell over-proliferation in Drosophila.Environ Sci Pollut Res Int. 2020 Jul;27(20):25261-25270. doi: 10.1007/s11356-020-08899-7. Epub 2020 Apr 28. Environ Sci Pollut Res Int. 2020. PMID: 32347502 Free PMC article.
-
The Role of Pea3 Transcription Factor Subfamily in the Nervous System.Mol Neurobiol. 2025 Mar;62(3):3293-3304. doi: 10.1007/s12035-024-04432-w. Epub 2024 Sep 13. Mol Neurobiol. 2025. PMID: 39269548 Review.
-
Shared Genetic Loci Between Body Mass Index and Major Psychiatric Disorders: A Genome-wide Association Study.JAMA Psychiatry. 2020 May 1;77(5):503-512. doi: 10.1001/jamapsychiatry.2019.4188. JAMA Psychiatry. 2020. PMID: 31913414 Free PMC article.
-
MIGGRI: A multi-instance graph neural network model for inferring gene regulatory networks for Drosophila from spatial expression images.PLoS Comput Biol. 2023 Nov 8;19(11):e1011623. doi: 10.1371/journal.pcbi.1011623. eCollection 2023 Nov. PLoS Comput Biol. 2023. PMID: 37939200 Free PMC article.
References
-
- Houben K, Nederkoorn C, Jansen A. Eating on impulse: the relation between overweight and food-specific inhibitory control. Obesity. 2014;22:6–8. - PubMed
-
- Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–850. - PubMed
-
- Keck PE, McElroy SL. Bipolar disorder, obesity, and pharmacotherapy-associated weight gain. J Clin Psychiatry. 2003;64:1426–1435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

